Verification vs. Validation: Decoding the Essential Differences for Product Success

Understanding Verification vs. Validation in Product Development
Definitions and Distinctions
Developers should possess an understanding of how and where verification and validation (V&V) fit into a QMS for a successful development of their product. Verification occurs during the development phase to ensure adherence to design specifications through inspections and testing. Conversely, validation takes place following the development phase to confirm that the product aligns with user requirements through methods such as user testing and clinical trials, The following article will explore how both processes play distinct roles in ensuring a product is well built and suitable for its intended purpose at different stages of the development process.
Various regulatory bodies have their interpretations of verification and validation, impacting how global companies approach product launches. The table below illustrates how the FDA, EMA and MHRA define these concepts:
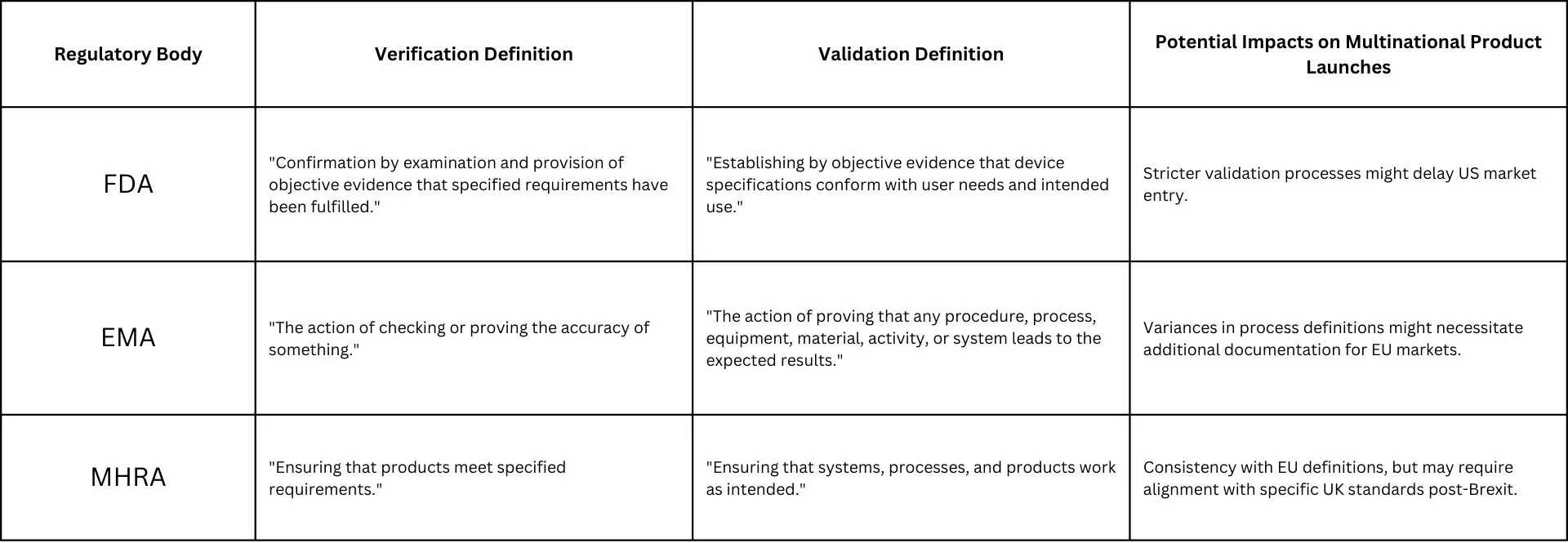
Understanding the distinguishing factors among these regulatory bodies is fundamental for launching products, for the variations in definitions and standards may cause delays in paperwork and approvals, ultimately hindering market entry. Tailored compliance strategies may be required for each regulatory body, impacting resource allocation. Although global alignment efforts can facilitate product launches, they depend on coordination among regulators.
Confusion in understanding verification and validation in the development process has presented challenges historically. For verification testing in particular, examples of noteworthy incidents can be observed NASA’s Mars Climate Orbiter and the Samsung Galaxy Note 7. NASA’s Mars Climate Orbiter failed due to a unit conversion error, resulting in a $125 million loss. The Samsung Galaxy Note 7 launched with battery issues and led to a $5 billion setback. These cases highlight the significance of verification in ensuring safety and functionality.
The Role of Verification and Validation in Regulatory Compliance
Securing approval from regulatory authorities for devices can be a lengthy and tedious process, but it is paramount to ensuring safety. Implementing V&V protocols can help expedite this process, allowing new innovations to enter the market quickly. The following are some examples of devices that adopted this strategy:
- Abbott BinaxNOWTM COVID-19 Ag Card
- Medtronic MiniMed 780G insulin pumps
- iRhythm Zio Patch monitors
- Intuitive Surgical da Vinci System robotic surgery systems
- Dexcom G6 continuous glucose monitors
While instances demonstrate how efficient V&V processes can reduce time-to-market and ensure availability of medical technologies, companies should consider common pitfalls that developers may encounter in V&V processes that can lead to compliance factors.
Developers may overlook the specific regulatory standards to which their product must adhere. These diverse standards entail varying technical requisites, often influencing fundamental decisions regarding device architecture. Failure to comprehend the applicable standard early in the development process can result in significant errors, necessitating extensive backtracking and redesign efforts.
Conducting internal testing in accordance with regulatory standards is also advised. Some companies opt to bypass comprehensive internal testing, relying instead on external testing agencies for validation. By conducting in-house testing, particularly for more high-risk assessments or pre-scans, developers can identify failures sooner before a third party test agency catches them.
Diving Deeper into Product Validation
Goals of Product Validation
The main goal of product validation is to make sure that the final product serves its intended purpose effectively. This involves testing the product in real-life scenarios to ensure it aligns with user expectations. This is especially necessary when developing medical devices.
Developers should conduct user research to incorporate anticipated user needs and expectations. This includes interviews and surveys with both healthcare professionals and patients to understand the potential requirements. Engaging limited users in a more controlled manner early in the design process will help inform user needs during the formal validation process. As a follow-up, developers should create user personas and scenarios to capture a range of needs that can be integrated into the validation process.
Assessing the success of a product validation phase can be measured using metrics such as detection rate, time taken to resolve defects, feedback from User Acceptance Testing (UAT) customer satisfaction ratings, regulatory compliance levels, and reporting events. These metrics can assess whether the product meets its intended purpose and regulatory standards, ensuring safety and effectiveness for users.
Methods and Stages of Product Validation
Ensuring that a product adheres to validation requirements at various stages is critical to its success. Validation is first addressed in the design phase, where proposals are evaluated for feasibility and implementation. As devices are assessed against validation criteria during the early prototyping phase, developers might identify any problems or deficiencies in the equipment. Finally, the end product is thoroughly validated to ensure that it meets user requirements and regulatory requirements.
Throughout these stages, various techniques can be employed to effectively validate the product. These techniques can include usability testing, field testing, and utilizing user feedback forums. Usability testing entails the examination of user interactions with the product to discern usability issues or areas of discomfort. Field testing allows a product to be tested in the real world, providing valuable insight into performance and durability. User feedback forums can gather input directly from users to further refine the product.
Due to regulatory requirements, validation steps vary between markets, particularly between consumer electronics and medical devices. For consumer electronics, the validation procedures lack standardized steps. Therefore, certain consumer device enterprises may omit validation activities entirely. Start-up ventures in consumer electronics may engage in minimal validation, typically consisting of informal Q&A sessions with prospective consumers and limited beta prototype testing. Conversely, larger consumer electronics corporations approach validation testing with strict formality, adopting methodologies akin to those observed in the medical device industry.
Regulatory agencies provide strict guidelines for validation of medical devices, including clinical trials and risk assessments that are not normally required for electronic device applications. Understanding these differences is important to determine the validation process needed for a specific device.
The Verification Process in Detail
Planning and Implementing Verification Tests
Verification tests ensure that the medical device meets its design specifications and requirements. Based on the product specifications, these tests will define objectives, procedures, and acceptance criteria. Validation tests can include inspection, testing, and analysis. Inspection involves a visual inspection or review of documentation, while testing verifies the functionality of the equipment. Analysis uses mathematical models or simulations to assess behavior.
Advancements in recent years have significantly elevated the efficacy of verification strategies, such as:
- Automated testing tools to simplify testing, ensuring comprehensive coverage
- Simulation software that enables realistic virtual testing environments, increasing accuracy
- Combining AI and machine learning algorithms to enable a faster analysis of large data sets, improving the detection of issues
- Agile development methodologies to encourage iterative testing and maintenance throughout the development process, increasing product quality and reliability
Designing verification tests to fit evolving specifications requires a flexible approach. This can include the following strategies:
- Ongoing review and communication to ensure alignment with changing requirements
- Using a modular testing framework, allowing for easy customization of specification updates
- Regression testing to verify that the variable does not cause random error
- Documenting changes and feedback for transparency on previous steps
- Risk assessment to prioritize testing efforts
- Joint decision-making to maintain equality of stakeholders
- Balancing a flexible test plan that can be adapted for evolving expectations
By following these steps, companies can effectively validate products, ensuring they meet user requirements and regulatory standards.
Challenges and Best Practices in Verification
Implementing verification in product development can lead to a number of challenges such as the standardization of tests and maintenance of complex specifications and requirements. These obstacles can prompt delays and increased costs if not properly addressed.
Implementing best practices can help identify and resolve issues early, reducing rework and delays. Establishing a continuous improvement cycle ensures that verification methods are based on feedback and new ideas while remaining consistent against required specifications. Furthermore, encouraging interdisciplinary collaboration and the use of automated testing tools can streamline validation efforts, leading to more efficient and cost-effective product development.
Integrating Testing & Validation into Product Development
The Importance of Early Testing
Integrating testing and validation from the outset of product development is critical for achieving success. Ideally, testing should be instituted during the initial stages of product conception and continue iteratively. By integrating testing and validation into each phase of product development, companies ensure that issues are identified and addressed promptly, minimizing their impact on timelines and budgets.
The benefits of early testing and validation are manifold. It provides immediate feedback on design decisions, enabling rapid iteration and refinement. Early testing can also reduce the likelihood of costly defects and failures later in the development lifecycle and enhance overall product quality and reliability, leading to improved customer satisfaction and market success.
Postponing testing elevates the likelihood of defects remaining undetected until advanced stages, where rectification could incur greater costs and time investment. This can result in project delays, budget overruns, and compromised product quality. Delayed testing may also lead to regulatory compliance issues if requirements are not adequately validated early on.
Tools and Technologies for Effective Verification and Validation
Leveraging advanced tools and technologies in V&V processes will lead to improved accuracy and efficiency, as various software and platforms enhance the overall effectiveness of these efforts.
Simulation and modeling tools play a significant role in validating and verifying complex systems. They allow engineers to simulate real-world scenarios and predict how a device will perform under different conditions. By conducting virtual tests, companies can identify potential issues early in the development process.
Industry-standard tools for validation and verification include:
- MATLAB/Simulink: Used for simulation and modeling in various industries, including medical device development
- Ansys: Provides comprehensive simulation solutions for structural, thermal, fluid dynamics, and electromagnetic analyses
- National Instruments LabVIEW: Enables engineers to create custom test and measurement systems for validation purposes
- IBM Rational DOORS: Facilitates requirements management and traceability, ensuring alignment between specifications and verification activities
- XRAY Plug-in for JIRA: Test management system for requirements, test documentation, and traceability
The latest advancements in V&V strategies involve integrating artificial intelligence (AI) and machine learning (ML) technologies. AI and ML algorithms analyze vast amounts of data to identify patterns and trends, improving validation efficiency and accuracy. These technologies automate test case generation, analyze test results, and predict potential failure modes. Ultimately, they can enhance overall testing effectiveness.
Incorporating AI and ML into validation and verification strategies can streamline testing processes, reduce manual effort, and provide deeper insights into product performance. This proactive and predictive approach leads to higher-quality products and faster time-to-market.
References
- https://sterlingmedicaldevices.com/thought-leadership/do-you-know-the-difference-between-verification-and-validation
- https://flairpharma.com/validation-and-qualifications
- https://startup-house.com/glossary/what-is-mobile-application-testing
- https://www.urbanpro.com/ansys/which-is-the-best-book-to-learn-ansys-workbench/37268868
- https://fatfinger.io/meeting-changing-demands-in-consumer-products-manufacturing
- https://www.bitstudios.com/blog/quality-assurance-in-software-development
- https://usvalidation.com/kb/validation_protocol_fr.aspx





