Commercialization Strategy
We share your mission: to get your device into the hands of those who need it. We help you avoid pitfalls and visualize the pathway to a successful product launch.
Our Evidence Based Commercialization Approach
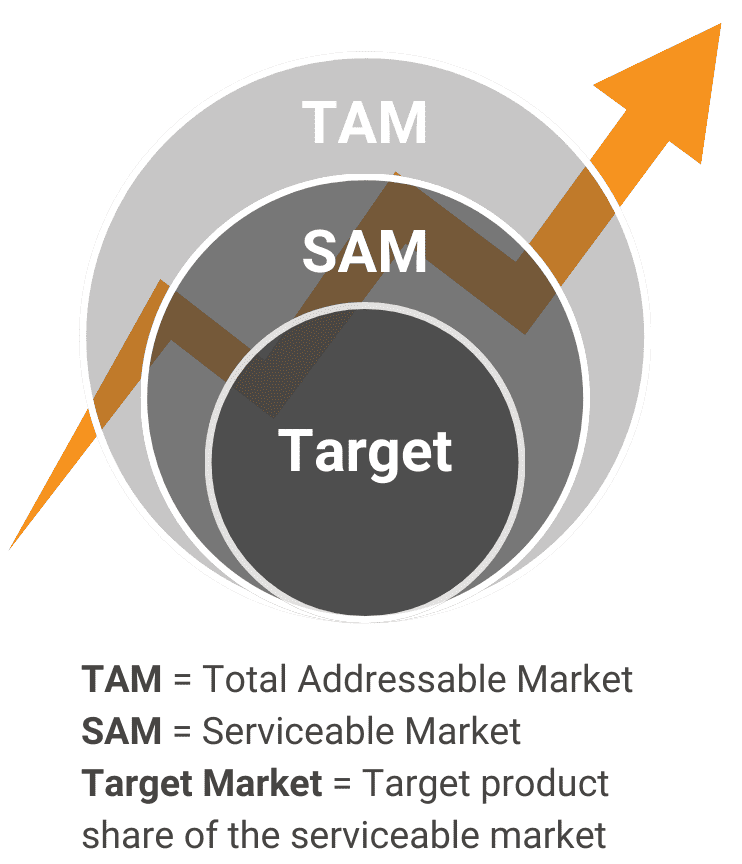
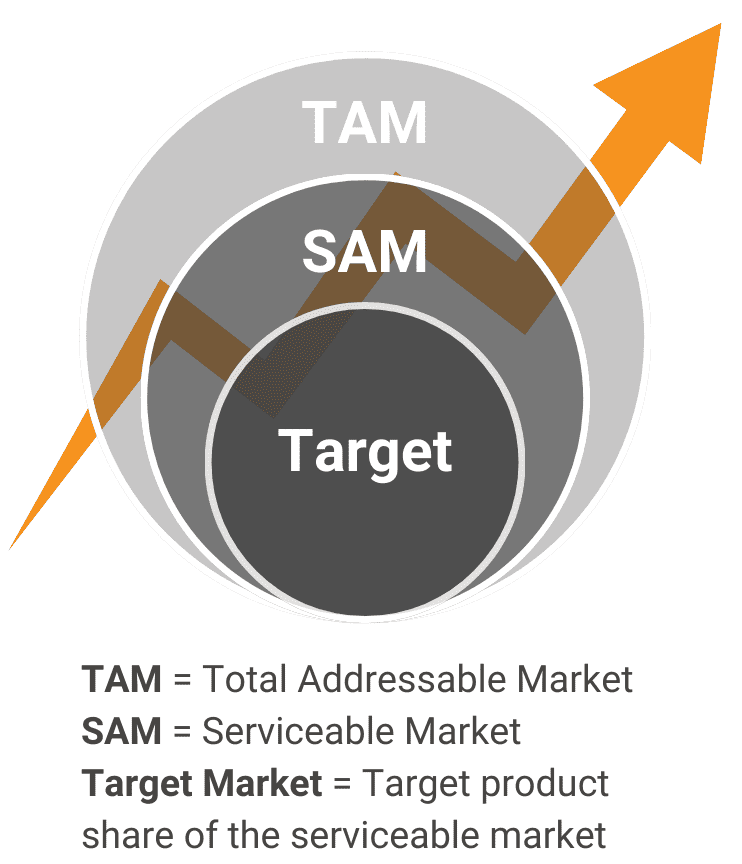
Is there sufficient demand for this product in the real world?Problem DefinitionWe describe the pain points and consequences that a customer would experience if they didn’t have your innovation. Market SizingWe quantify the market volume, growth, and trajectory from the top down and from the bottom up. Market SegmentationWe define the subsections within existing customer groups (considering divisions caused by opinions, attitudes, geography, socioeconomic factors, and barriers) |
 |
Who is the target customer and what motivates them to buy the product?Early AdoptersWe help you pinpoint the customer groups who will be first in line to buy and test out your product. Key Opinion LeadersOur team maps out the professional organizations and individuals with the most influence over your target market. Launch OptimizationWe formulate a plan for initial sales to the target customer and subsequent expansion to additional market segments. |
   |
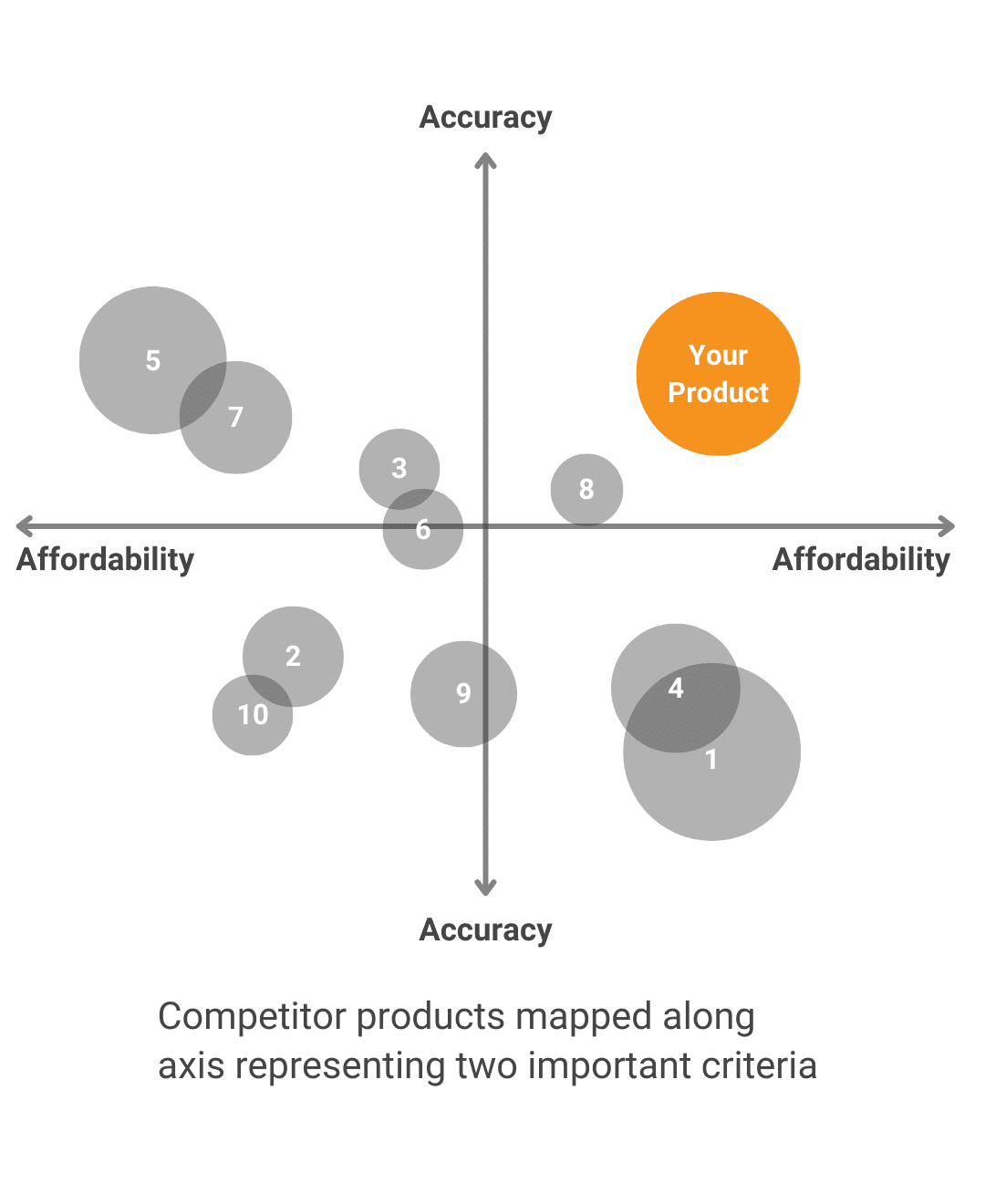
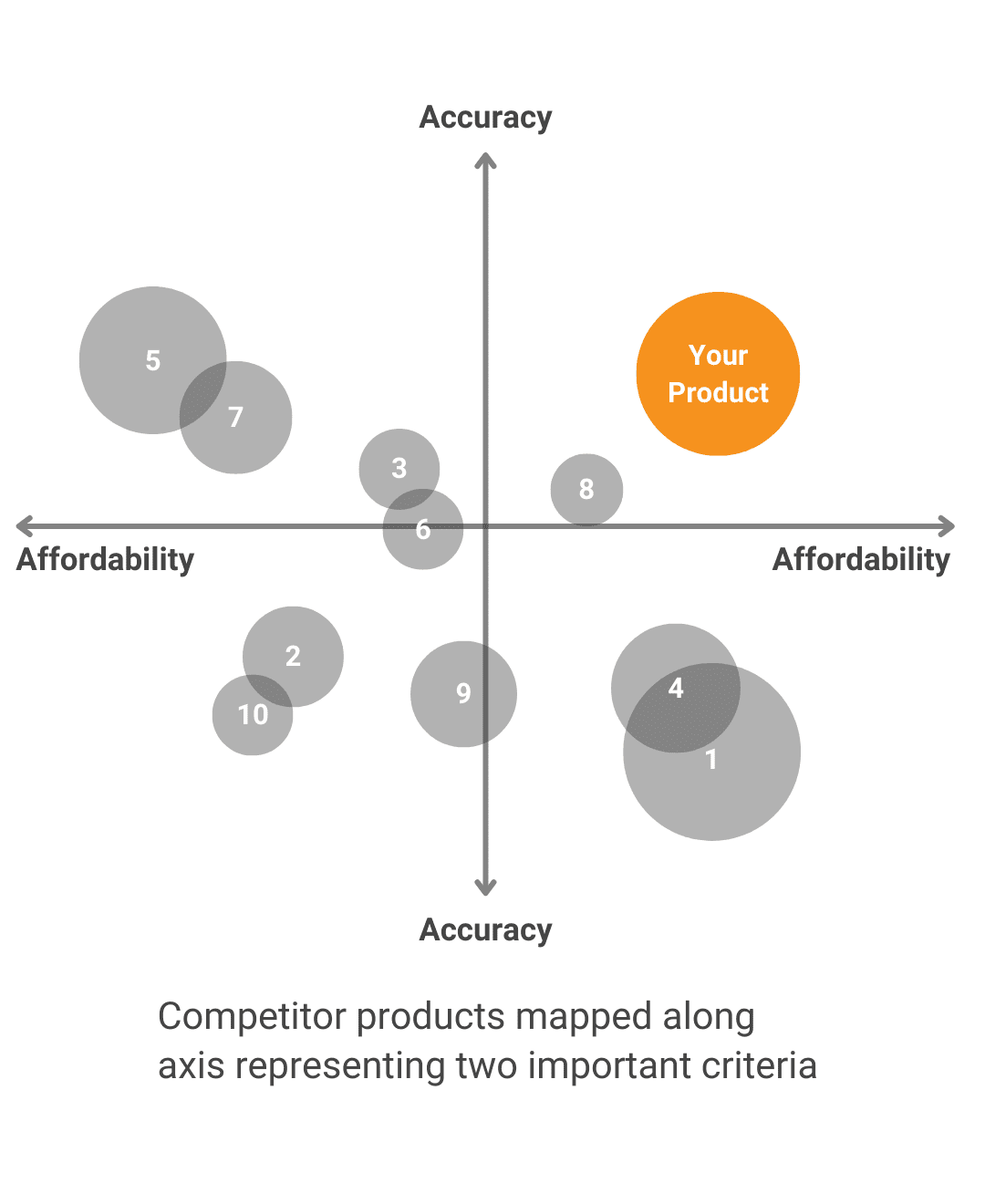
What other options exist to solve the problem and what makes your solution the better one?Technology SWOTWe objectively evaluate the innovation’s strengths, weaknesses, opportunities and threats Alternative SolutionsWe analyze the other choices available for solving the same problem. Value DifferentiationWe work with you to develop a value proposition that highlights the unique strengths of your product and how it aligns with stakeholders’ needs. |
   |
What proof of effectiveness is required by key stakeholders?Current Practice StandardsWe help you understand the status quo and gauge the resistance to change among potential users of your product. Care Pathway & WorkflowWe describe the journey of each stakeholder’s interaction with the product along the continuum of care. Evidence PortfolioWe examine the appropriate depth and level of rigor for testing the product concept and the product itself. |
 |
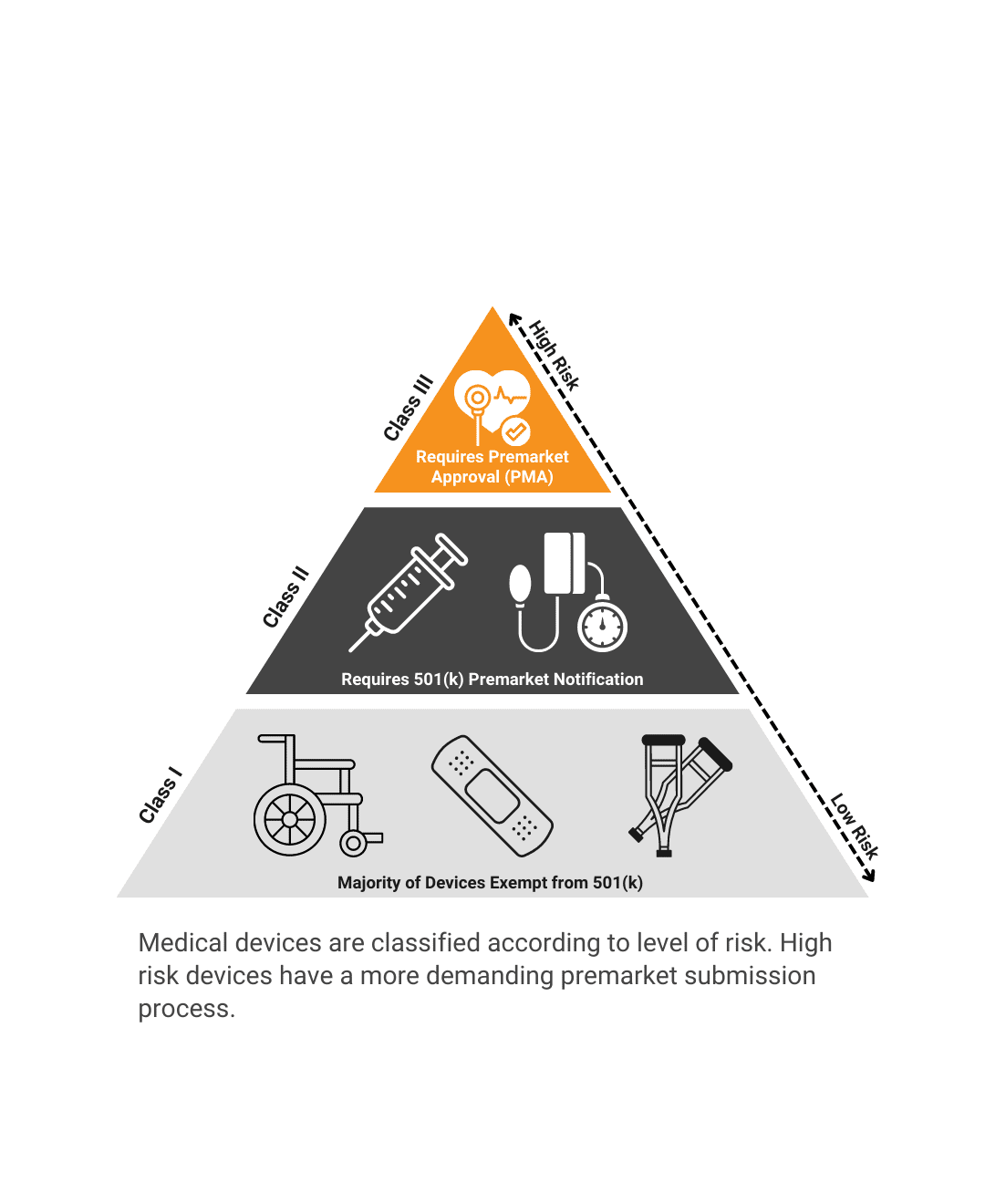
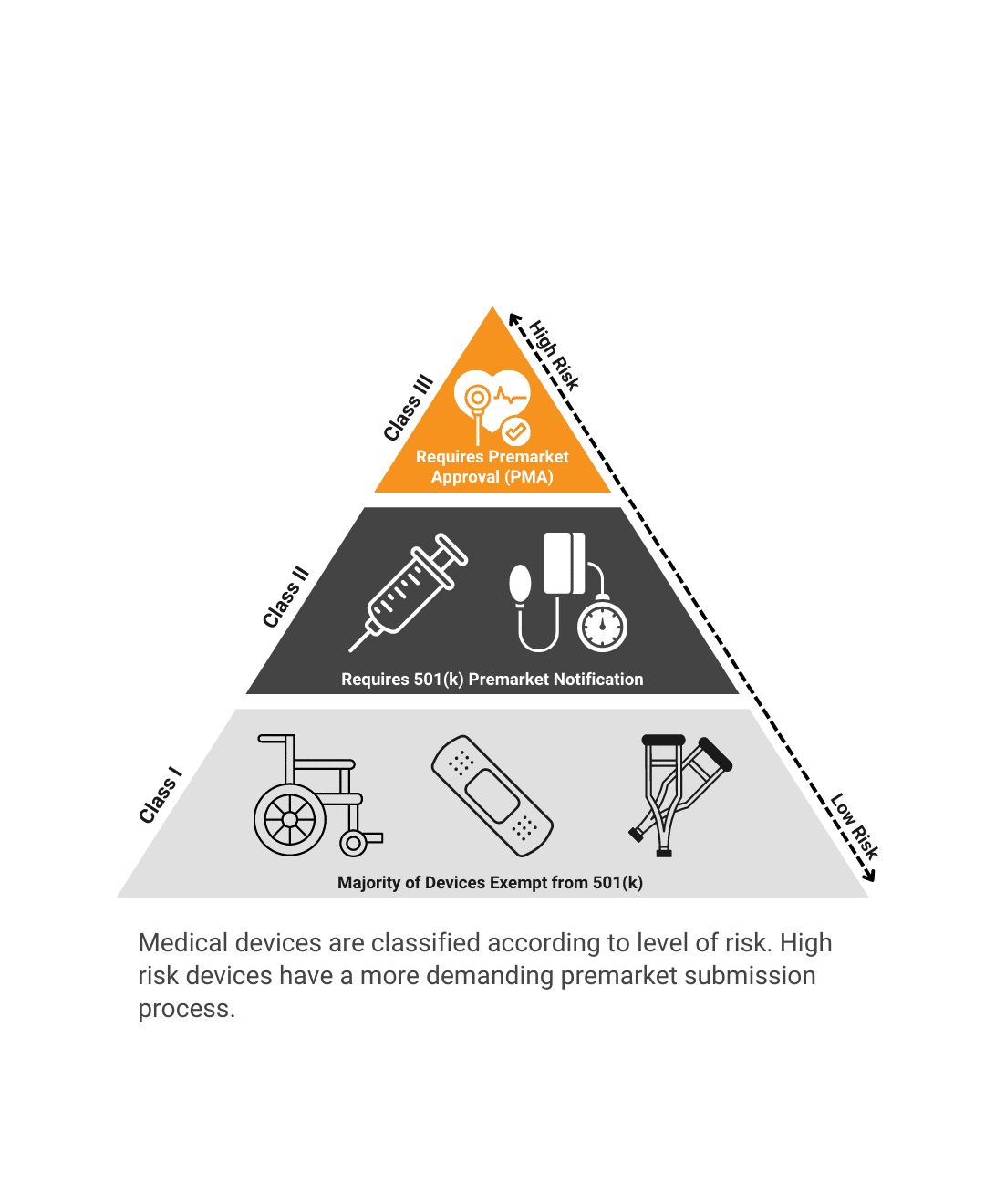
What regulatory barriers must be cleared before this product launches?FDA Submission PathwayWe help you reduce risk by outlining future regulatory requirements. Quality RequirementsWe recommend a strategy for documentation and good manufacturing processes. Industry StandardsWe identify product testing requirements and recommend a verification and validation plan. |
   |
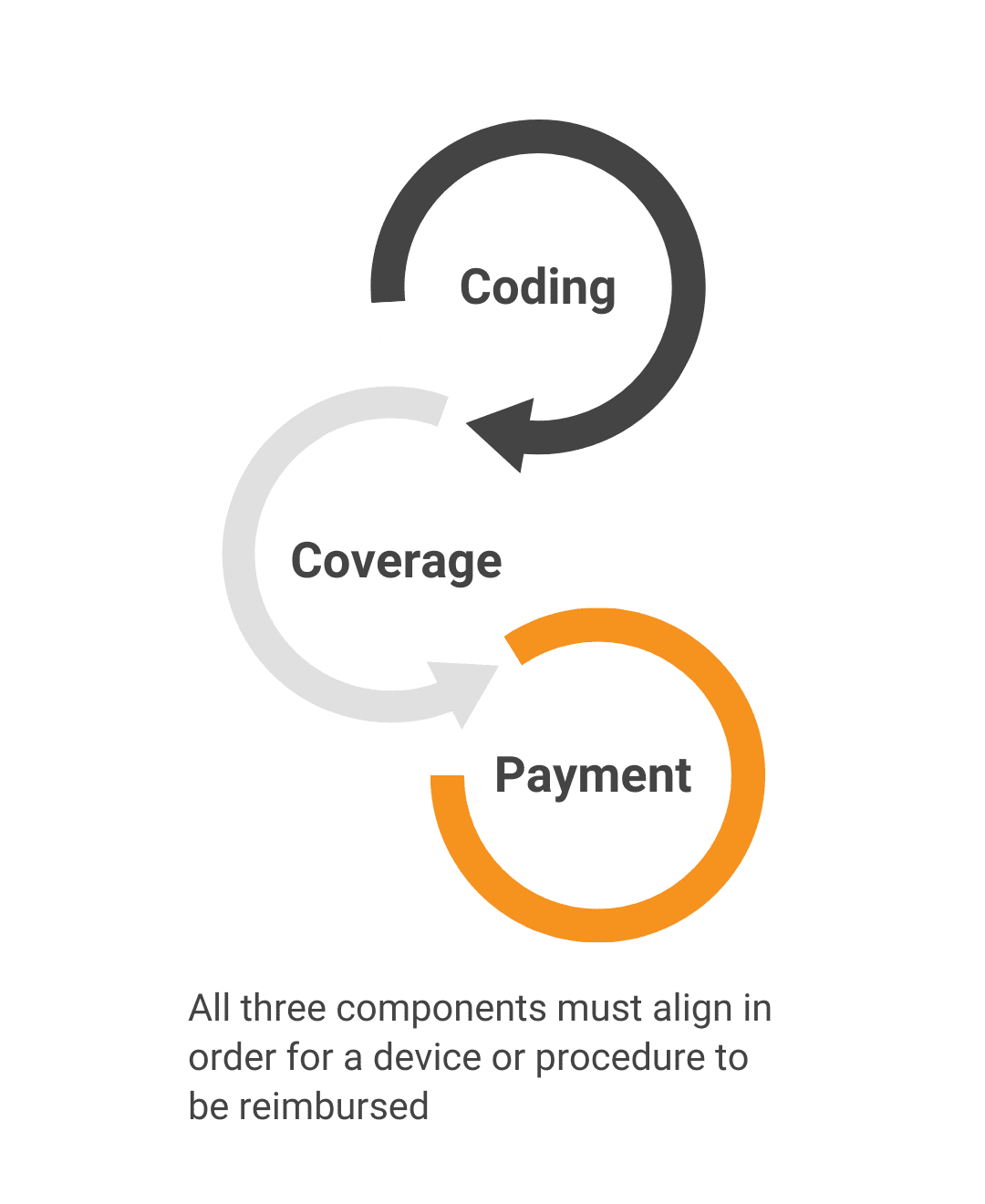
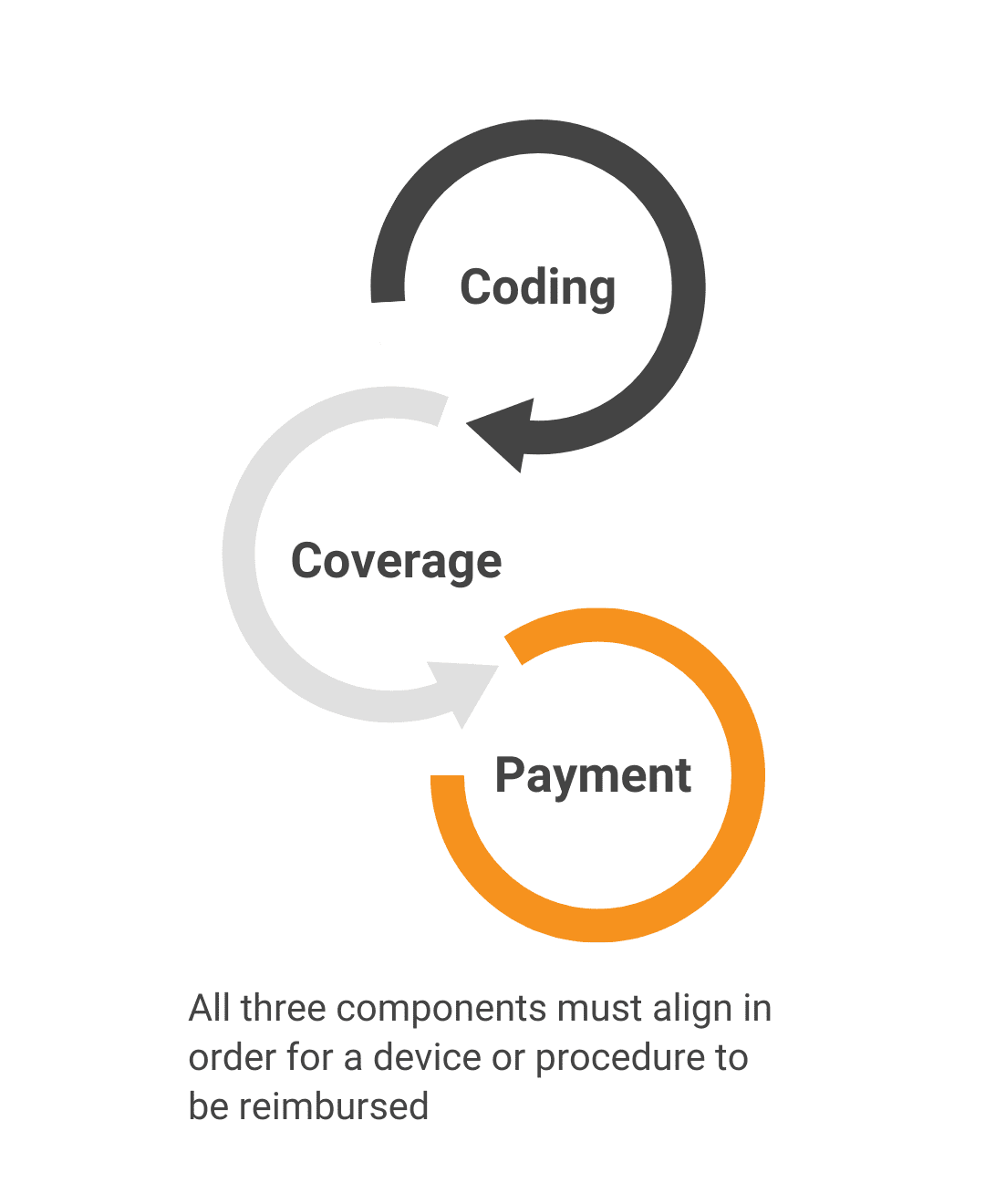
Will the patient’s health insurance pay for this product?Benefit Category/CodingWe determine where your innovation fits within the existing medical insurance framework. Coverage PolicyWe review existing policy determinations and justifications to predict whether your product will be considered reasonable and necessary for reimbursement. Payment & PricingWe evaluate how much payers are willing to reimburse and compare that against what stakeholders are willing to pay. |
   |
Is there sufficient demand for this product in the real world?
Problem Definition
We describe the pain points and consequences that a customer would experience if they didn’t have your innovation.
Market Sizing
We quantify the market volume, growth, and trajectory from the top down and from the bottom up.
Market Segmentation
We define the subsections within existing customer groups (considering divisions caused by opinions, attitudes, geography, socioeconomic factors, and barriers)


Who is the target customer and what motivates them to buy the product?
Early Adopters
We help you pinpoint the customer groups who will be first in line to buy and test out your product.
Key Opinion Leaders
Our team maps out the professional organizations and individuals with the most influence over your target market.
Launch Optimization
We formulate a plan for initial sales to the target customer and subsequent expansion to additional market segments.



What other options exist to solve the problem and what makes your solution the better one?
Technology SWOT
We objectively evaluate the innovation’s strengths, weaknesses, opportunities and threats
Alternative Solutions
We analyze the other choices available for solving the same problem.
Value Differentiation
We work with you to develop a value proposition that highlights the unique strengths of your product and how it aligns with stakeholders’ needs.



What proof of effectiveness is required by key stakeholders?
Current Practice Standards
We help you understand the status quo and gauge the resistance to change among potential users of your product.
Care Pathway & Workflow
We describe the journey of each stakeholder’s interaction with the product along the continuum of care.
Evidence Portfolio
We examine the appropriate depth and level of rigor for testing the product concept and the product itself.

What regulatory barriers must be cleared before this product launches?
FDA Submission Pathway
We help you reduce risk by outlining future regulatory requirements.
Quality Requirements
We recommend a strategy for documentation and good manufacturing processes.
Industry Standards
We identify product testing requirements and recommend a verification and validation plan.



Will the patient’s health insurance pay for this product?
Benefit Category/Coding
We determine where your innovation fits within the existing medical insurance framework.
Coverage Policy
We review existing policy determinations and justifications to predict whether your product will be considered reasonable and necessary for reimbursement.
Payment & Pricing
We evaluate how much payers are willing to reimburse and compare that against what stakeholders are willing to pay.



We Are Your Partner
We offer expert consultation and education to help you achieve your product and business goals.
We’ve Been in Your Shoes
Our team leverages a commercialization methodology developed over two decades of bringing our own and client’s technologies to market.
We Provide Real-World Solutions
Our team equips you to bring your innovation from prototype to commercial product. We emphasize product-market fit, so that your device works as well in the real world as it does in the laboratory.
500+ Clients from Startups to Fortune 500 Companies
Success Stories:

Why Simbex?
Simbex combines industry leading product design, engineering, commercialization, regulatory, and reimbursement expertise with an established production-pathway and clear market access, to provide a holistic ideation-to-market solution.
Our Commercialization Leadership Team


Angela Presley, PhD
Director, Commercialization Services


Karen Page, PhD
Senior Research Analyst


Haley Schwartz
Consultant































