Medical Device Software, App & Web Development
Software Development Services
Software development continually evolves with increasing complexity and integration of new technologies to help keep pace with customer expectations. We have a diverse group of developers to cover the needs of users from embedded firmware to mobile apps and cloud-based solutions.
We utilize an agile approach to software development to ensure development remains on track, and customer needs and requirements are met. Our development teams and quality assurance processes ensure each project is “rightsized” with a focus on delivering the right software at the right time and with the appropriate level of verification/validation from unit testing to integrated systems testing.
Looking for Assistance with a Medical Device Software Application?
"*" indicates required fields
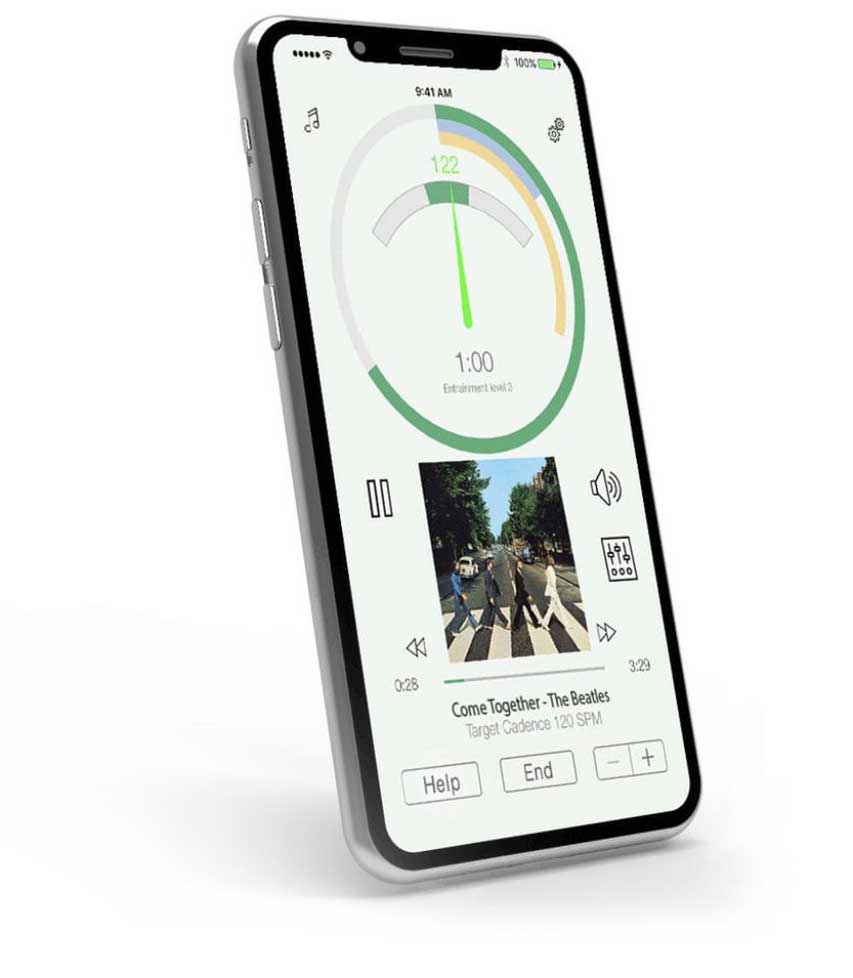
Internet of Things (IoT)
In the medical device field, Internet of Things (IoT) describes the system of physical technologies which relay information to other devices, and often refers to functions such as data collection, patient monitoring, and research analysis.
Known as the Internet of Medical Things (IoMT) in this application, it has also played an important role in improving the connections between available healthcare resources and patients in need of medical care. As telehealth applications are increasingly used in diagnosis and treatment, IoMT has led to a rise in the idea of “Smart Healthcare” – technology designed to create a more streamlined and automated healthcare system.
IoMT is most frequently used for remote monitoring and emergency notification systems, through the use of both wearable devices and specialized sensors in a person’s living space. While these types of devices are often developed to assist patients undergoing a treatment or procedure, they are also commonly applied to people living with chronic diseases and disabilities, the elderly, and people with mobility impairments.
With the assistance of remote monitoring devices, healthcare workers and loved ones can monitor a patient’s well being. These devices include:
- Thermometers
- Electrocardiography (ECGs)
- Electroencephalography (EEGs)
- Cardiac monitors
- Apnea monitors
- Spirometers
- Oximeters
- Audiometers
- Blood pressure monitors
- Breathing frequency monitors
- Electronic stethoscopes
Advances in design and production have enabled the development of ultra-low cost, single use IoMT sensors, saving upwards of an estimated $300 billion in annual healthcare costs. These are often made of high-grade medical polymers and fabricated on paper or e-textiles to create wireless powered, affordable and disposable sensing devices.
Portability and low system-complexity are key aspects of a successful device, as IoMT-based systems are patient-centered and must cater to their medical conditions. You will also need the proper programming to support your device. As IoTs are becoming a continuously larger part of the healthcare system, the software that connects devices and manages data is equally as important to creating a market-ready product. Our software engineers have the experience and capability to ensure your IoMT product is program-compatible and ready to perform in the field.
Medical Device Regulatory Compliance
Regulations often change and complicate a product’s compliance. In addition, the individual requirements for each aspect of a device and its components can vary from area to area. This increasingly complex system of laws and regulations makes it difficult to understand which restrictions apply to your device and its programming, as well as whether or not you meet the standards set in place by different entities. In order to make sure your device complies with local and international regulations, it is important to perform a thorough regulatory review and risk assessment.
We’ve Supported 550+ Awesome Clients
Cybersecurity Through Secure Software Development
The future of healthcare relies on accessibility and user-friendly technology. As patients and medical professionals increasingly manage their electronic health information (EHI) over the internet, the threat of a cyber attack is heightened. A medical device collecting protected health information must have the proper security to protect it, as data breaches are becoming more sophisticated. Healthcare organizations frequently suffer from account takeovers, bot attacks, and ransomware, which can place the wellbeing and personal information of patients at risk.
With the threat of online attacks and malware on the rise, both private and governmental entities around the world are rapidly tightening the restrictions on what software is approved for use on the market. As a developer of a new medical device, it is important to take cybersecurity very seriously, as protecting both the functionality of medical equipment and the personal information of patients is essential. The software your device relies on to operate will need to have the ability to meet the requirements set in place by both the CISA and organizations abroad and as defined in the following regulations: UL-2900-1:2017, IEC 80001-2-8:2016, IEC 80001-2-2-2012. When developing your product’s software, it is crucial to ensure it complies with all applicable regulations and will be ready to pass evaluations to make it to market.

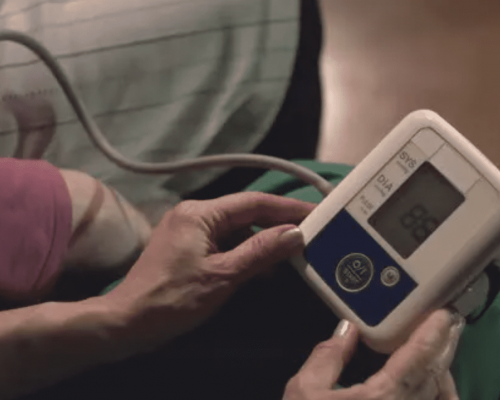
Biometric Sensors
Biometric sensing and monitoring requires not only proper sensors to capture biological information; it also requires understanding user intent or user context. Any data your sensors collect relies on software and algorithms to record and model biomarkers in a such as your oxygen levels, blood pressure, and heart rate. This is accomplished through the use of Biometric software/middleware. Biometric data is crucial to monitoring the wellbeing of patients, and can be used to track variations in health, detect diseases early-on, as well as functioning as an emergency warning system. In situations where healthcare workers are monitoring at-risk patients, a functioning and accurate identification system can be the difference between life and death. Simbex’ team understands the variables that must be considered when choosing the right sensors to gather accurate biometric data.
Sensor Data
Our focus at Simbex continues to be on helping users realize the value of wearables and the data that they collect with innovations in two main areas: understanding context awareness and user intent, and understanding how sensors, embedded hardware, and algorithms work in harmony to provide value to the user.
In recent years, the use of IT tools in medicine has rapidly expanded, digitizing many aspects of the healthcare system. As technology progresses and advancements such as wearable devices and remote monitoring systems become commonplace, so do the data processing systems and patient databases required to manage the vast inputs of information.
Health monitoring systems process and analyze a patient’s data that it has received from technology such as smartphones, wristbands, as well as various connected sensors and wearable devices. However, collecting data without understanding user intent or user context makes any data we collect have questionable value. The Simbex approach is to use as much readily available information to help provide some level of context awareness. When possible, we integrate intrinsic data from sensors on a user along with supplemental data from other sources. These external sources could be current and historical information from calendars (what people are doing or have done) or beaconing systems/location tracking from phones (understanding where people are or have been) coupled with ideas from the field of proxemics.

Simbex is proud to be recognized as a Nordic Design Partner!
Simbex uses Nordic Semiconductor for the wide range of available capabilities and development tools (including nrfConnect), and the combined Bluetooth and peripheral capabilities on a single platform, streamlining our workflow for the connected smart sensing devices we develop.
Those looking to develop a device within this market are presented with the opportunity to create a revolutionary product at the forefront of medical advancements, aided by the ever-growing need to improve the healthcare system. A novel device, equipped with effective software, can be transformative to both patients and medical practitioners.
Ready To Get Started?
What happens when you combine technical product development expertise with a commercialization mindset? A straighter path to market, fewer roadblocks or surprises, customers that become evangelists and investors that become follow-on investors. That is what Simbex brings to the table.


















