DHF vs DMR vs DHR: Everything You Need to Know

Medical device development is a highly regulated process. Maintaining meticulous documentation throughout is a regulatory requirement and crucial for ensuring product safety and efficacy. Among the critical documents that medical device companies must manage are the Design History File (DHF), Device Master Record (DMR), and Device History Record (DHR). Each serves a unique purpose in the lifecycle of a medical device, from concept to production.
What Are DHF, DMR, and DHR?
DHF, DMR, and DHR each describe a set of documents that serve a unique purpose in the lifecycle of a medical device, from concept to production. It’s important to understand the differences between these essential documents and their roles and follow best practices for managing them effectively.
Definition of DHF (Design History File)
The Design History File (DHF) is a comprehensive documentation collection that details a medical device’s evolution from the initial concept to the final design. It ensures the device has been developed in compliance with FDA regulations and meets the intended user needs.
While several ways exist to create a comprehensive DHF documentation process, the Waterfall Design Controls Process adapted from Health Canada by the FDA in 1997 remains common. Other methods, such as the V-model1, have gained popularity in systems engineering and software development for medical devices
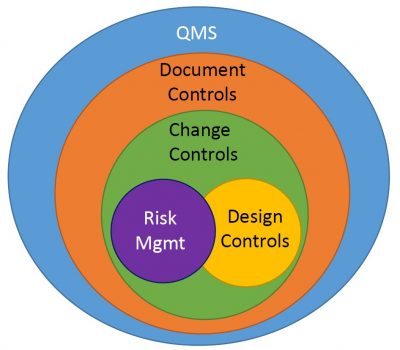
Figure: Application of Design Controls to the waterfall design process. From Design Control Guidance for Medical Device Manufacturers2 by the FDA (1997)
Essential Components of the Design History File (DHF)
The FDA does not prescribe a specific structure for the DHF, but industry best practices often organize it into User Needs plus the eight Design Control sections outlined in 21 CFR 820.30.
Note: All Class II and Class III (and some Class I) medical devices require Design Controls to be in place for FDA approval.
User Needs
User Needs guide the entire development process, ensuring the final product is safe, effective, and user-friendly. These needs, identified through interviews, observations, and usability studies, are documented in the DHF to ensure they translate into actionable design inputs, guiding the rest of the development process.
For instance, if the intended users are emergency medical responders who need to administer medication efficiently during chaotic situations, a user need might be: “The device must provide quick and easy access to medication while attending patients.” This user need would then translate into specific design inputs.
Design Plan
The design plan outlines the design process’s scope, objectives, and critical activities. It calls out roles and responsibilities, ensuring that everyone understands their part in the project. The plan is dynamic and often updated as the project progresses.
Design Inputs
Design inputs are a device’s physical and performance requirements derived from user needs, regulatory requirements, and industry standards. Proper documentation ensures the design addresses all necessary specifications.
Following the emergency medication example, one design input could be “The device must have a one-handed operation mechanism.” Think of a narcan applicator, this would address the user need.
In reality, several design inputs go into solving the same user need. For example, “the applicator must be able to be stored in a pocket” could be an additional design input.
Design Outputs
Design outputs are the results of the design efforts, including drawings, specifications, and manufacturing instructions. They must align with the design inputs and are crucial for creating the DMR.
Design Review
The purpose of a design review is multifaceted: it helps identify any concerns in the design and development stages, ensures that the design is appropriately reviewed at critical points, and includes the necessary representation from all relevant stakeholders.
These reviews evaluate the adequacy of the design requirements and assess the capability of the design to meet these requirements. Additionally, design reviews serve as the first line of defense in risk management by identifying and addressing potential problems early in the process.
Design Verification
This step confirms that the design outputs meet the design inputs. Verification activities like tests and inspections are documented to prove the design is correct.
Design Validation
Validation ensures that the final device meets user needs and intended uses, often through real-world testing. Proper documentation is critical to demonstrate that the device performs as intended. Common validation techniques include:
-
Clinical Trials:
Testing the device in a real-world healthcare setting to observe its performance under actual conditions. -
Simulated Use Testing:
Using the device in a controlled environment that mimics real-life scenarios. -
Usability Testing:
Evaluating the device’s ease of use and user interactions to ensure it meets the intended user requirements.
-
Environmental Testing:
Assessing the device’s performance under various environmental conditions, such as temperature and humidity, to ensure it remains functional in different settings.
These techniques help confirm that the device operates as expected and meets all safety and effectiveness criteria.
Design Transfer
Design transfer involves moving the design to production, including creating manufacturing processes and quality control procedures. Documentation of this process ensures a smooth transition.
Design Changes
Changes are inevitable in the development of medical devices, and the DHF must document all design changes, including their rationale, impact, and implementation. Effective management of these changes is a critical component of the Quality Management System (QMS).
Before initiating Design Control processes, robust Change Control and Document Control processes must be established. These processes are heavily influenced by risk management practices and require clearly defined Standard Operating Procedures (SOPs) to ensure consistency and compliance. The QMS helps maintain detailed version records, ensuring that every change is traceable and well-documented.
These controls are applicable to all device types—whether hardware, software, or a combination of both—and play a vital role in maintaining the integrity and safety of the final product. The graphic below illustrates how these controls are interrelated and essential to the overall design process.
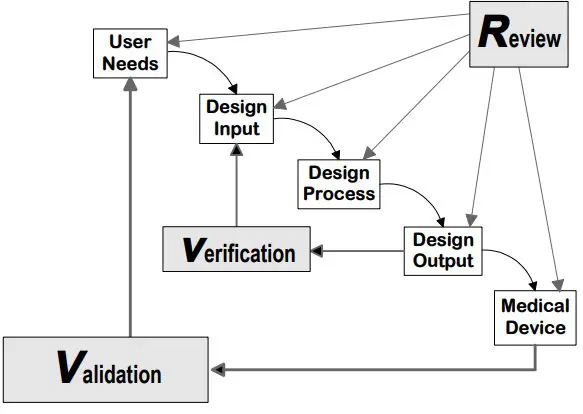
Figure: Graphical representation of how design control elements of the DHF fit within a QMS.
Definition of DMR (Device Master Record)
The Device Master Record (DMR) compiles all the information necessary to produce the medical device. It is the manufacturing blueprint containing detailed instructions on building, testing, packaging, and labeling the device. This ensures that every device produced is identical in quality and performance.
The DMR typically includes the device’s specifications, manufacturing processes, quality assurance procedures, packaging and labeling requirements, and installation, maintenance, and servicing procedures. By maintaining an up-to-date DMR, manufacturers can verify that all production processes are controlled and standardized.
Understanding the Roles of DHF, DMR, and DHR in Medical Device Development
How Are DHF, DMR, and DHR Related?
While DHF, DMR, and DHR each serve distinct roles in the medical device lifecycle, they are closely interrelated and form a comprehensive documentation system that ensures the device is designed, manufactured, and tested following all regulatory and quality requirements.
- The DHF captures the design process, ensuring the device meets the necessary design inputs and user needs.
- The DMR translates these design outputs into a manufacturing blueprint, ensuring that every device is produced according to the design specifications.
- The DHR documents the production process, providing traceability and proof that each device was made correctly.
Together, these documents ensure that every aspect of the device’s lifecycle is documented—from initial design to final production. This comprehensive documentation is crucial for meeting regulatory requirements and ensuring the device’s safety and efficacy.
What Are the Regulatory Requirements?
In the United States, the FDA’s Quality System Regulation (21 CFR Part 820) outlines the document requirements for medical devices, specifically for the DHF, DMR, and DHR. Specific sections for each are listed in the table below. These documents must be maintained and updated to ensure compliance with these regulations. When submitting a medical device to the FDA, you must show evidence of the entire software development life cycle, such as documentation, processes, and test results.
In addition to FDA regulations, international standards play a crucial role in ensuring global compliance. ISO 13485:2016 provides a framework for quality management systems specific to medical devices, while ISO 14971:2019 offers guidance on risk management processes.
Furthermore, IEC 62366 focuses on the application of usability engineering to medical devices. This standard ensures that medical devices are designed with the intended user in mind, reducing the likelihood of human error and improving overall safety and effectiveness. Compliance with these standards is essential for medical device companies operating in multiple markets.
Comparing DHF, DMF, and DHR
Understanding the unique scope and purpose of the DHF, DMR, and DHR is crucial for ensuring that a medical device is designed correctly, manufactured, and documented in compliance with regulatory standards.
| Design History File (DHF) | Device Master Record (DMR) | Device History Record (DHR) | |
|---|---|---|---|
| Primary Focus | Design and Development | Manufacturing Blueprint | Production History |
| Purpose | To document the evolution of the medical device design from concept to final design. | To provide detailed instructions on how to manufacture the device. | To document the production history of each device |
| Required Documents |
|
|
|
| Required Documents |
|
|
|
|
|||
Follow Best Practices for Managing DHF, DMR, and DHR
Managing the DHF, DMR, and DHR effectively is key to maintaining compliance and ensuring consistent product quality. Here are some best practices.
How to Maintain and Update These Files
The DHF should be updated throughout the design and development process, especially after design reviews and changes. The DMR should be updated whenever there are changes to the manufacturing process. The DHR should be updated in real-time during manufacturing to ensure accurate and complete production records.
How to Create and Organize Documentation
Utilize digital tools for document management to streamline the process of maintaining DHF, DMR, and DHR. Standard Operating Procedures (SOPs) should clearly define roles, responsibilities, and methods for managing these documents, ensuring consistency and compliance.
How to Ensure Compliance and Quality Control
Regular audits of DHF, DMR, and DHR are critical. These audits should check for completeness, accuracy, and traceability and be conducted at each significant stage of the device lifecycle. Training is also vital to ensure that all team members understand the importance of these documents and are proficient in maintaining them.
Overcome Common Challenges in Documentation
Managing medical device documentation can be challenging, but with the right strategies, it’s possible to maintain accurate, up-to-date records that comply with regulatory requirements.
How to Maintain Accurate and Up-to-Date Records
Implement regular check-ins and reviews throughout the design and manufacturing process. Use digital tools to automate parts of the documentation process, reducing the risk of human error and improving accuracy.
Common pitfalls include failing to document changes promptly, inconsistencies between design and manufacturing records, and incomplete records. To avoid these, establish clear guidelines for documentation and ensure that all team members are trained to follow them.
How to Integrate Documentation Systems
A unified documentation system integrating DHF, DMR, and DHR can streamline record management and improve traceability. Digital solutions should be carefully planned to ensure accurate migration of existing records and compliance with regulatory requirements.
Explore the Future of Medical Device Documentation
As technology continues to evolve, so does the medical device documentation landscape. The future promises new tools and methods for managing DHF, DMR, and DHR more efficiently and effectively.
Tools and AI for Documentation
New digital tools are continually emerging that can help streamline the documentation process. These tools can offer features like automated version control, real-time collaboration, and enhanced traceability, making it easier to manage complex documentation requirements.
Artificial Intelligence (AI) is beginning to play a role in medical device documentation, offering the potential to automate more complex tasks such as risk analysis and document reviews. AI-driven tools also help identify gaps in documentation, ensuring that all regulatory requirements are met.
Key Takeaways
-
Critical Documentation:
DHF, DMR, and DHR are essential documents in medical device development, each serving a specific role in ensuring the device is designed, manufactured, and documented according to regulatory standards. -
Importance of Accuracy:
Accurate and comprehensive documentation is crucial for regulatory compliance and ensuring the safety and effectiveness of medical devices. Proper DHF, DMR, and DHR management is vital to achieving these goals.
-
Best Practices for Compliance:
By following best practices in managing these documents, medical device companies will be well-prepared for regulatory inspections and audits, reducing the risk of non-compliance and enhancing product quality.



