Key Concepts for Medical Device Entrepreneurs
Lessons Learned from Simbex’s Federally-Funded Medical Device Commercialization Centers
At Simbex, we work with healthcare startups, established medical companies, medtech entrepreneurs, and other innovators to help bring their device concepts to life. We’ve been designing and developing medical devices and health and wellness products for clients, as well as bringing our own innovations to market, for over twenty years!
Our holistic approach to product design centers on identifying risk at every stage of the commercialization process from concept to market and planning ahead to lower the level of risk as much as possible. We never say “We’ll worry about that when we get there!”
What you might not know about Simbex is that we’ve honed this approach over a decade of running innovation commercialization programs for the National Institutes of Health and the Food and Drug Administration. Both NIH and FDA recognized the importance of helping medical device entrepreneurs navigate the commercialization pathway, particularly the early market assessment and business model development milestones that are often overlooked or underestimated for their importance.
They awarded grants to Simbex to help guide innovators in the rehabilitation and assistive technology space through our NIH-funded Center for Translation of Rehabilitation Engineering Advances and Technology (TREAT) and in the pediatric medical device space through the FDA-funded New England Pediatric Device Consortium (NEPDC). Simbex continues to advise biomedical entrepreneurs through the NIH-funded DRIVEN Accelerator Hub.
Simbex Has Provided Assessment & Advisement on More Than 900 Medical Device Concepts
Through these federally supported consortia, grant funds were thoughtfully allocated to develop impactful programming, providing education, project assessment, and financial as well as in-kind assistance to reach as many innovators as possible.
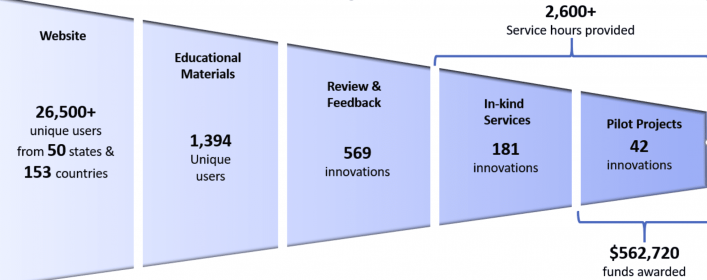
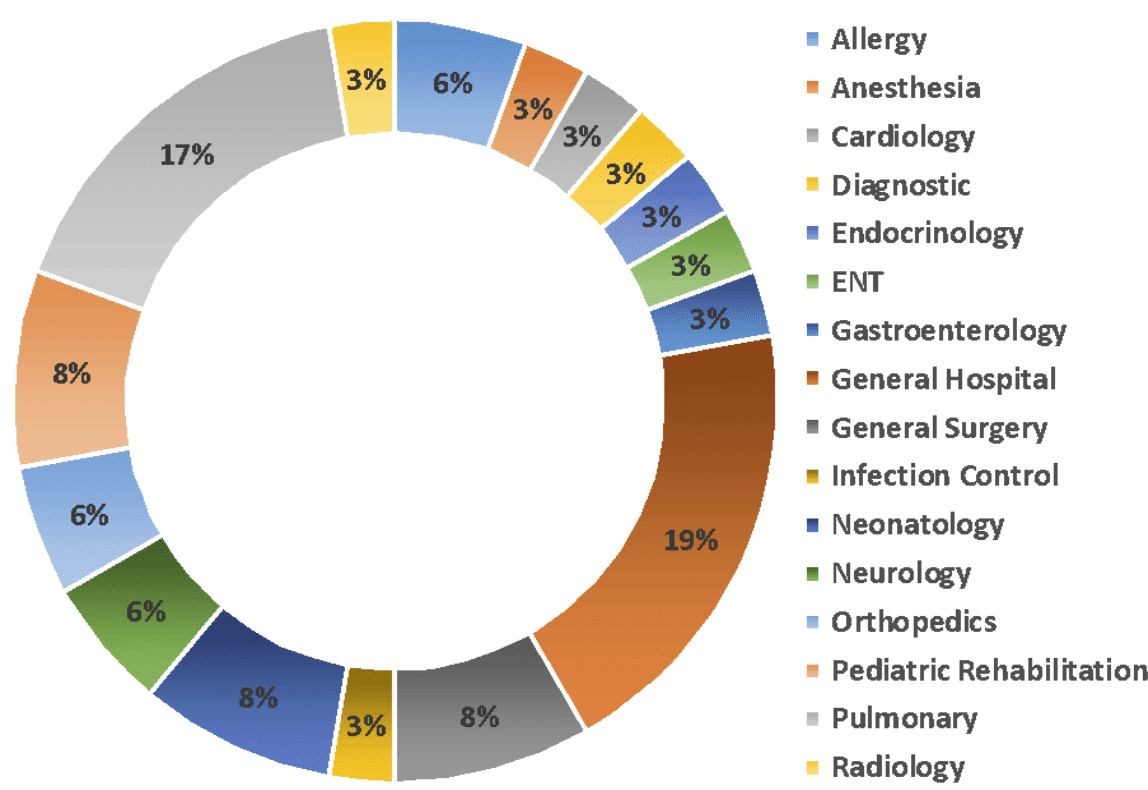
Across all 900 device concepts, the same challenges were presented to us again and again by the innovators who engaged with our commercialization centers, reinforcing the need for the programs and guidance we provided. Below are some of the most common topic areas we covered, which remain at the heart of the commercialization consulting services we provide today.
The Top Six Key Concepts for Medical Device Entrepreneurs:
1.
Embrace a structured approach to identify and plan for both hurdles and opportunities. Understanding and then systematically achieving each milestone will positively affect a product’s market viability and increase likelihood of success.
2.
Always take the time to determine the market for your product and to understand the needs and wants of target customers before further developing the technology. Think through the key product capabilities that the market deems critical for addressing the problem or need; this is the basis for developing your Market Requirements Document.
3.
Understand who your stakeholders are, and how your product and business model will address their needs. Customer discovery is not only a great tool to verify a market need for your device, it will help you test your assumptions with all your stakeholders as you progress along the commercialization pathway.
4.
Take steps to determine whether an opportunity exists for your medical device product idea. Some questions to consider: Do you have an understanding of your particular industry and how it operates? Will your product fit nicely into an existing clinical pathway or will it be disruptive? Do you have Freedom to Operate?
5.
How do you envision earning revenue? Spend some time vetting the business model for your market. This includes researching sales, purchasing and distribution channels, identifying potential licensees or partners, fundraising strategies, and more.
6.
When the reimbursement and insurance system is not well aligned with the goal of the device, payment will be a large hurdle to overcome on the path to commercialization. It is essential to understand who will be buying your device and how they will be paying for it.
If you need assistance in any of these areas, let the Simbex Commercialization Services team know you would like a no-obligation call to discuss how we could help you further your Medical Device Business goals:
"*" indicates required fields






