Simbex Works with Career Clinician to Realize His Vision for an O&P Wearable Device
Seeing the Need
Dennis Clark has had a lifetime of experience in orthotics and prosthetics (O&P). He trained under his father and eventually took over his practice, growing it from a small family business to a multi-site enterprise, Clark and Associates, headquartered in Waterloo, IA. Highlights of his career include serving as the president of ABC (American Board of Certification) and holding appointments as lead prosthetist at Walter Reed Medical Center during the conflicts in Iraq and Afghanistan.

Like many healthcare professionals, while Dennis observed the profession from every angle throughout his career, he noticed that there was an unmet need.
There was no patient-specific data on how patients actually used their O&P devices during activities of daily living. As the importance of evidence-based practice on patient outcomes became prevalent in other areas of medicine, the need for objective outcome data in O&P became obvious. For example, clinicians had many questions about the patient’s usage patterns when they left the clinic with their new prosthetic leg.
- Did they use the prosthesis full time, or only for certain activities, such as when they left the house, and were only using crutches at home?
- What if the patient wasn’t using their prosthesis at all, and it was just sitting in the closet collecting dust?
- How would the medical team know about this pattern of behavior and be able to anticipate and address any potential adverse outcomes?
Even the very best O&P devices are only capable of creating functional change in a patient’s life if that person actually wears them.
Dennis decided that one way to fix this lack of information would be to add a sensor to the patient’s O&P device that would be capable of capturing patient data in the real world, similar to other wearable devices.
He experimented with various sensors that were available on the market, but he kept running into problems. They were too bulky, too heavy, too expensive, and the battery life was too short.
He knew that these factors would cause user friction that might influence the patient not to wear their O&P device because of the cumbersome sensor that was attached to it.
Trials & Errors
After several years of trial and error on his own, his frustration mounted. Not only were the sensors not-quite-right, but getting them had its own share of challenges, such as having to purchase in bulk when all he needed was a few for experimentation.
Vendors weren’t really set up to work with him, and he struggled to get the support he needed to solve the problem. And working with the sensors themselves to try and put together a proof of concept wasn’t as straightforward as it should be.
There was a seemingly unending and growing list of things that needed to be figured out. He needed help from someone who understood his vision to make it a reality.
It was at this point that Dennis met Simbex and began to experience the power of partnership.
Video: Early Form Factor & Functional Prototype. Note the empty plastic tab and mount above the Simbex prototype sensor, which demonstrates the huge difference in size and profile of earlier prototypes which Dennis describes as the “Bad Times” before Simbex was involved.
There were three aspects to working with Simbex that were deciding factors for him:
“First off, Simbex had the unique ability to listen and really hear what I was trying to do, and also had the depth of knowledge across the board engaged in that process.
Secondly, after they understood what I was trying to accomplish, they kept me within some parameters, so I didn’t try to do too much too fast.
And Third, I sat in the room and not only heard, but watched the thought process and how fluid they were in picking up what I was trying to say and rolling that into what we were doing. It just flowed seamlessly from person to person with everyone bringing their expertise to solve my problem.
That sealed the deal for me.”

Dennis Clark, CPO | Founder OPOS1
Understanding the Opportunity with a Comprehensive Expert Team
Given our experience with commercializing products, we understand that it pays great dividends to begin with the end in mind and clarify where the product fits in the marketplace before developing the technology. Having both commercialization and technical capabilities working together under one roof is a powerful combination. Therefore, our first project with Dennis was a commercialization strategy project to make sure he was on the right track.
We listened to his product vision and assessed the market needs and opportunity, regulatory and reimbursement pathways, and competitive technologies. “Simbex did a tremendous job of tearing my thought process down to a root level and starting over, and that was very, very important. If we were going to work together, they wanted to know exactly what I was trying to accomplish and why I wanted to accomplish it.”
We determined that the field of O&P was not the only area that could use a wearable sensor to track medical device usage patterns; there were also opportunities in orthopedics, podiatry, wound care, and other market segments. Because this market size was so much larger than Dennis’ original use case, we encouraged him to move ahead with his project, but to keep flexibility in mind so that his final product would be suitable for a variety of uses. A serendipitous reimbursement development that occurred during the project was the addition of reimbursement codes for Remote Therapeutic Monitoring. These codes allow for Physical Therapists and Physicians to bill for their time when examining sensor data that is gathered remotely. Dennis’ sensor system fit the definition of this code set perfectly. This reinforced Simbex’s recommendation to move forward with developing the product.
Now that the commercial path was established, the next phase was a targeted engineering project to document the requirements of the sensor system. Similar to the commercialization strategy, there needed to be a clear vision of the technical development pathway. These requirements, defined in the Product Requirements Document, are foundational to medical technology development. Dennis had a short but challenging list of requirements. He wanted to develop a sensor that was capable of detecting movement so that it could be used to measure gait and wear time.
He also told us that ideally the sensor system should be “invisible, lightweight, and free.”
Simbex unpacked this list of requirements to see what we could realistically provide.

Lightweight
O&P devices are made to be as streamlined as possible, but they also have to be strong, so they are made of heavy-duty materials. An orthotist who works hard to design a brace with as little excess material as possible does not want to add weight to the device to accommodate a sensor.
A patient who already has to wear an orthosis on her leg does not want to feel unnecessarily weighed down. This meshes with the first requirement; the sensor should not impact the patient’s experience when wearing their O&P device.

Free
Dennis knew that reimbursement is always a pain-point in O&P. It can be very difficult for orthotists and prosthetists to get adequate payment from the patient’s health insurance to cover the costs of providing O&P devices. The sensor must not add to the financial burden for the orthotists / prosthetists or for the patients. Importantly, the orthotists / prosthetists should be able to follow their typical workflow and not have to schedule extra patient visits to accommodate the sensor. This means that the battery life on the sensor must be able to span the three to six months that typically elapse between appointments.
Build vs Buy?
With any Simbex project, the team determines whether to build vs. buy an existing system which meets the product requirements, and thus not “reinvent the wheel” if something already exists that will meet our client’s need. After we completely understood the requirements and product vision, the Simbex team evaluated any existing sensors to see if we could adapt these products for Dennis’ purposes.
- We scoured the market for low power micro sensors.
- We considered the entire span from data loggers meant for tracking industrial shipments to tiny IMUs that biologists strap to the back of honeybees for pollination research.
In the end, it was clear that Dennis’ use case required a customized sensor design.
Embarking on the Product Development Journey
Working together in an Agile methodology, Simbex assembled an interdisciplinary project team to design a sensor system with an accompanying mobile/web application and cloud database, while relying significantly on Dennis’ clinical and industry expertise.

Simbex leveraged our deep experience with wearable biometric sensors and gait biomechanics, along with our extensive component partner relationships, to develop a sensor design that incorporated a peel and stick application method suitable for retrofitting as well as for installation on a newly fabricated O&P device.
The shape resembles a plus sign with flexible wings that can be trimmed as needed and includes rivet holes as an alternate application method for use on fabric goods.
The enclosure is completely clear so that it comes very close to meeting the “invisibility” requirement.
The team was able to design the componentry and firmware to optimize battery life to achieve a remarkable six months of wear time on a single coin cell battery by completely understanding the intended use of the device. Rather than continuously or periodically transmitting data, a manual button was integrated to align with the clinical model.
As the project team grew, Simbex and the client worked together as integrated partners to form a complementary team of expertise that included systems architecture, software, firmware, electrical and mechanical engineers.
After a few rounds of prototyping, it began to take shape. Dennis named the product OPOS1 which stands for Orthotic Prosthetic Outcome System.
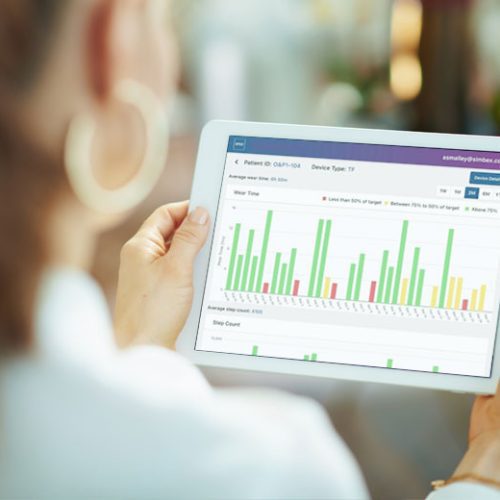
Simbex was also able to rely on our deep background in biomechanics analysis and gait measurement to develop a precisely tuned algorithm for the device.
Measuring wear time is remarkably efficient, given the customization for this application and the resulting elegant simplicity. For clinicians who do not use mobile devices, they can also access the patient data from their web browser.
Visualization was an important consideration in a field that is not used to dealing with data – the graphs display red, yellow or green bars to indicate if the patient is wearing their device according to their utilization targets.
The clinician is in charge of setting the targets and they can be adjusted as needed to accommodate a wide range of patient types.
The data is intentionally minimalistic, focusing on two main variables that are collected and displayed:
How many minutes per day did the patient wear their device?
How many steps per day did they take while wearing it?
If a patient doesn’t use their device for a few days in a row, they might receive a call from their clinician asking if something needs to be adjusted to make them more comfortable.
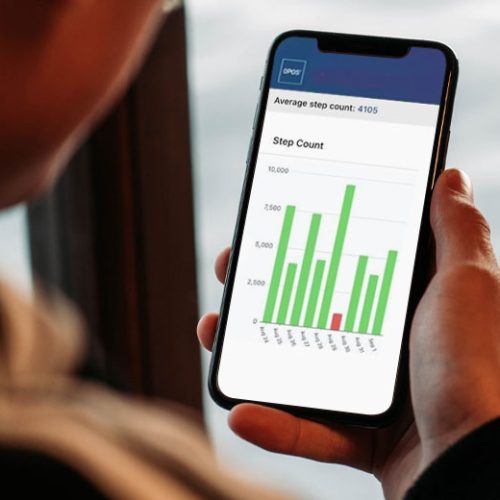
Patients can also log onto the app if they choose to. They can see their own data and take ownership of their outcomes.
Dennis knew from his personal experience of wearing a continuous glucose monitor that patients would feel empowered by this opportunity, and it would lead to behavioral change.
If the patient sees that their step count was low for a few days in a row, they might go for a walk so that they can get closer to their targets.
Motivated patients feel satisfaction in seeing a row of green bars indicating they have consistently been within 75% of their target utilization.
From Idea to Reality
In the space of about three years, Dennis took his idea from concept to reality with the help of the various highly specialized Simbex product teams working closely together. He launched OPOS1 at trade conferences and is currently working on a validation study with Mayo Clinic.
He is also coordinating with the Limb Loss and Preservation Registry to tie the data from OPOS1 to patient records within the registry. As product demand grew, the Simbex team evaluated several contract manufacturers to determine the best fit for production. Simbex then managed the relationship with the chosen contract manufacturer, and currently maintains the cloud database.
Dennis is seeing his goal of improved data in the field of O&P come to fruition. The team at Simbex is proud to be partnered with Dennis as part of this accomplishment!
“I was just amazed that that they could go from this little thought all the way to that point with a timeline that we’ve stayed pretty much on. It has been a journey, but they listen, and maybe that’s the most impressive thing. There was no question from the minute we started talking that I might have had this dream, but all the brains are at Simbex, and they listened so they knew that what they were creating was what I was really trying to do.”