FemTech is slated to be worth $1 trillion by 2027 | Here are 8 FemTech companies we are watching in 2024.
When it comes to investing in healthcare startups, the future is undoubtedly female. Currently, only 3% of healthcare investment deals are focused on FemTech, any technology that addresses health issues that disproportionately impact women. That leaves a vast untapped market that’s on the brink of explosive growth: FemTech is projected to be a $1 trillion industry by 2027.
“After 20+ years of developing products in the medical device space, it’s great to see the growth in innovation in this important sector. At Simbex, we are working with more and more FemTech companies as they develop novel tech-enabled products including wearable products, navigate reimbursement and regulatory challenges and develop their business plan and pitch deck.”
With that in mind, here are the top 8 companies we’re watching in the FemTech space — both for their innovative technology, and their ability to disrupt the market.
Reia Health
Their Value Prop: Allowing women to self-manage pelvic prolapse at home
More than half of women over the age of 50 experience pelvic prolapse, yet the condition is highly stigmatized and has seen little innovation in treatment over the last 50 years. Currently, treatment protocol involves surgery or a pessary, a device inserted into the vagina to support the pelvic floor.
The pessaries available today must be inserted by a medical professional, and require regular follow ups to clean and maintain the device. Reia Health will change that. The company has developed a pessary women can insert, remove and clean at home, thereby improving their quality of life, decreasing risk of complications from long-term wear, and increasing treatment accessibility to those who do not have regular access to physician care, according to Reia CEO Kaitlin Maier.
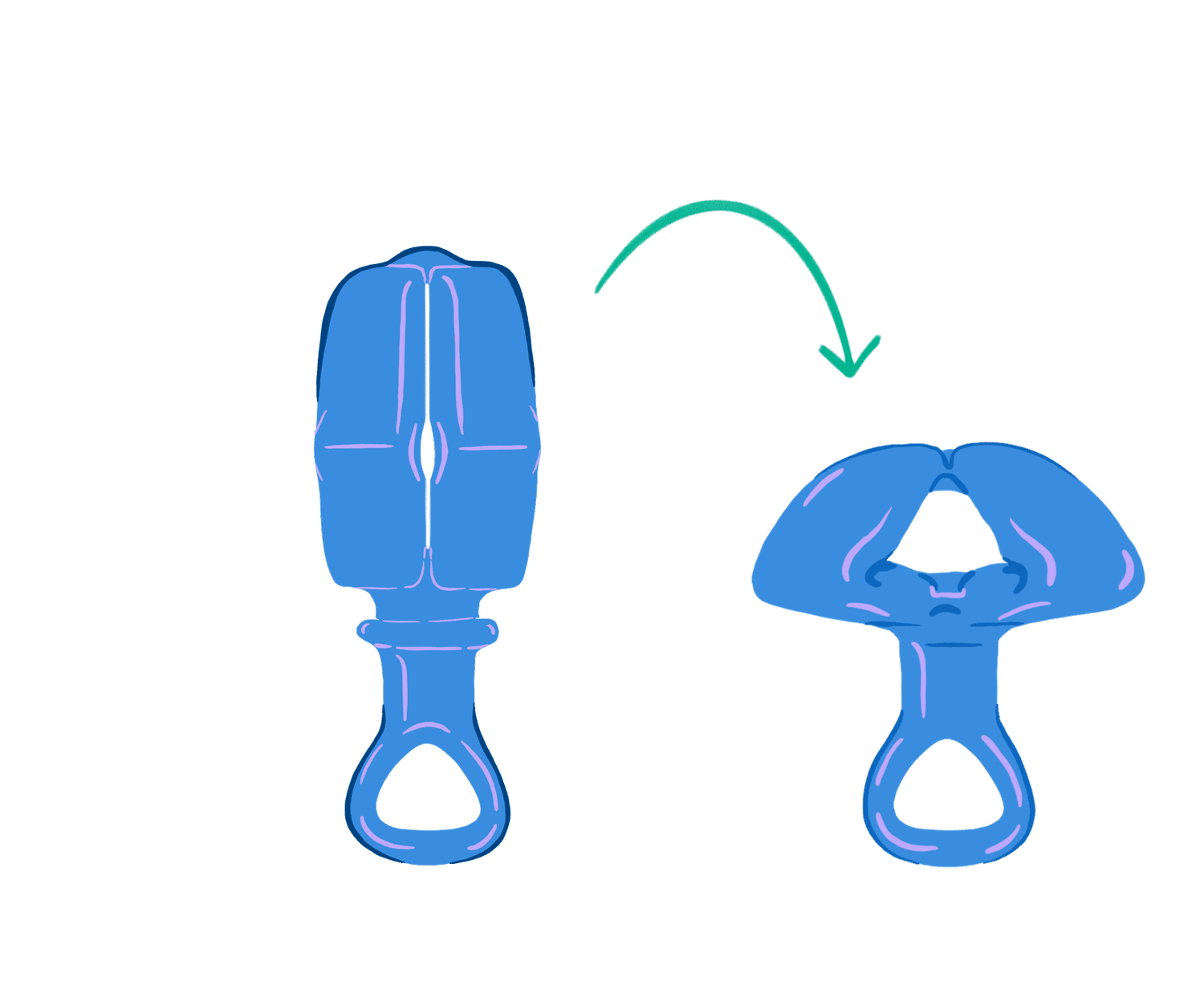
The Reia pessary is pending FDA approval, and Maier expects it to launch in the summer of 2024. Concurrently, the company will be running a clinical trial with the national Foundation for Female Health Awareness.
The result, Maier anticipates, will be not only a better patient experience, but a more efficient healthcare system.
“Reia’s pessary can reduce burdens on the healthcare system by reducing complications from consistent long-term wear and by freeing up the highly specialized physicians from pessary cleaning visits and allowing them to focus on higher acuity appointments,” she said.
Neuraura
Their Value Prop: Minimally invasive treatment for chronic conditions, starting with PCOS
Neuraura has developed thin-film electrodes, harnessed to create minimally invasive stimulation devices that will treat a variety of conditions, beginning with polycystic ovary syndrome (PCOS).
The company’s LoOop device uses Neuraura’s patented electrodes coupled with a TENS device to provide stimulation that regulates sex hormones. The result? Improved PCOS symptoms, including more regular periods, increased ovarian blood flow and reduction in blood glucose levels. Claire Dixon, co-founder and CEO of Neuraura, expects Looop to launch to consumers next year.

“We have developed a pre-production prototype and are now evaluating partners to take us to design freeze and small-scale manufacture,” Dixon said, adding, “We have proven commercial interest and already have a letter of intent for up to $1 million in pre-sales with a leading online pharmacy in Canada.”
But that’s just the beginning, according to Dixon. The company plans to fight diabetes through a disposable version of the Looop device; and treat conditions ranging from migraines to incontinence using neuromodulation. Overall, Dixon estimates the market opportunity at $5.7 billion, and the impact on health to be just as significant.
“Our purpose is to transform the standard of care for chronic, underserved conditions,” she said. “Our goal is to empower women to achieve optimal long-term health outcomes.”
Matricis.AI
Their Value Prop: Harnessing AI to get faster diagnoses for women’s health conditions, starting with endometriosis
Currently, women with endometriosis—a painful condition where uterine lining grows outside of the womb—wait an average of ten years to get an accurate diagnosis. Matricis.AI plans to cut that wait drastically, by using medical-grade artificial intelligence to assist radiologists in interpreting women’s pelvic MRI results.

The company has already partnered with Paris-based APHP, the largest hospital network in Europe. Now, they’re getting ready to expand into the American market, according to CEO Raphaelle Taub.
“The coming 12 to 18 months are pivotal for us, as we aim to expand our collaborations to the US and initiate the regulatory approval process,” she said.
Taub is operating with urgency, she said, to address the historical neglect of women’s health issues and to corner an often-overlooked segment of the market.
“The radiology AI market globally stands at approximately $20 billion, with half of that dedicated to MRI,” she explained. “Of the 400 FDA-approved AI tools for radiology, none specifically address women’s pelvic conditions. Given that gynecological diseases affect 6 in 10 women during their lifetimes, this represents a significant untapped market opportunity.”
Glooma
Their Value Prop: Using AI to diagnose breast cancer sooner
The co-founders of Glooma want to put the knowledge of 1,000 doctors into the hands of women. They do this using the SenseGlove, a device that women wear while examining their breasts. Sensors in the glove relay information on breast tissue to the Glooma app, which tracks changes month-over-month in order to identify any concerning growths.
The idea came after co-founder and CEO Francisco Neto Nogueira’s cousin was diagnosed with breast cancer. She had noticed a lump, but second guessed herself and wasn’t certain if she should see a doctor. Neto Nogueira’s father, a physician, said this is all too common, and contributes to why 37% of breast cancers are not detected early.
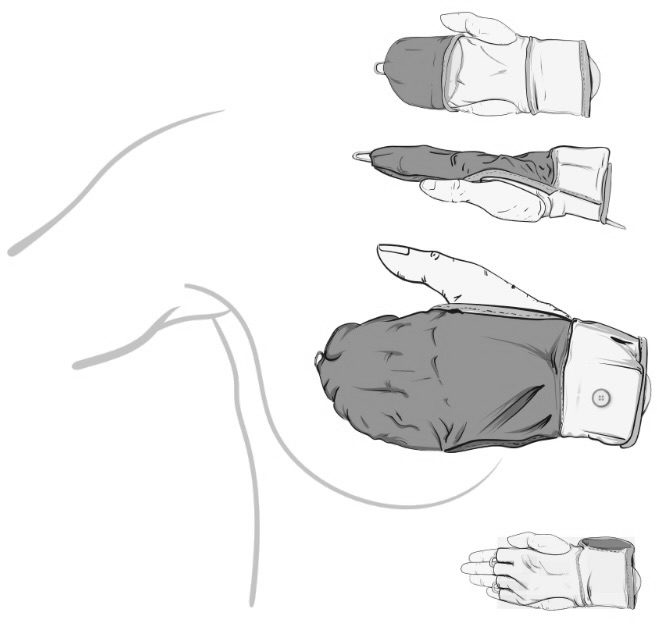
Breast self-exams can “create uncertainty and stress for women,” said co-founder Frederico Stock. “We want to give them peace of mind and more knowledge about their bodies.”
In 2024, Glooma will pursue FDA registration in the United States, and begin pilot programs with thousands of American women. The SenseGlove will be marketed directly to consumers, to insurance companies, and to major employees to incorporate into their employee benefit packages.
With assistance from AI, the SenseGlove will encourage women to connect with medical care sooner, when breast cancer is most treatable.
“We want women to take action sooner and reduce late detection breast cancer,” Stock said.
Armor Medical Inc.
Their Value Prop: Early detection of hemorrhage, one of the most costly and deadly maternal health complications
Armor Medical Inc. is developing a technology to aid in early detection of postpartum hemorrhage, one of the leading causes of preventable maternal death worldwide, including in the United States.
The company’s first device, Maternal Armor, is a bracelet that monitors blood flow patterns to detect hemorrhage well before other vital signs, like heart rate and blood pressure, begin to show distress. That early detection allows providers to treat hemorrhage with less invasive and less expensive measures, potentially saving the life of the patient and avoiding life-altering treatments like hysterectomies.
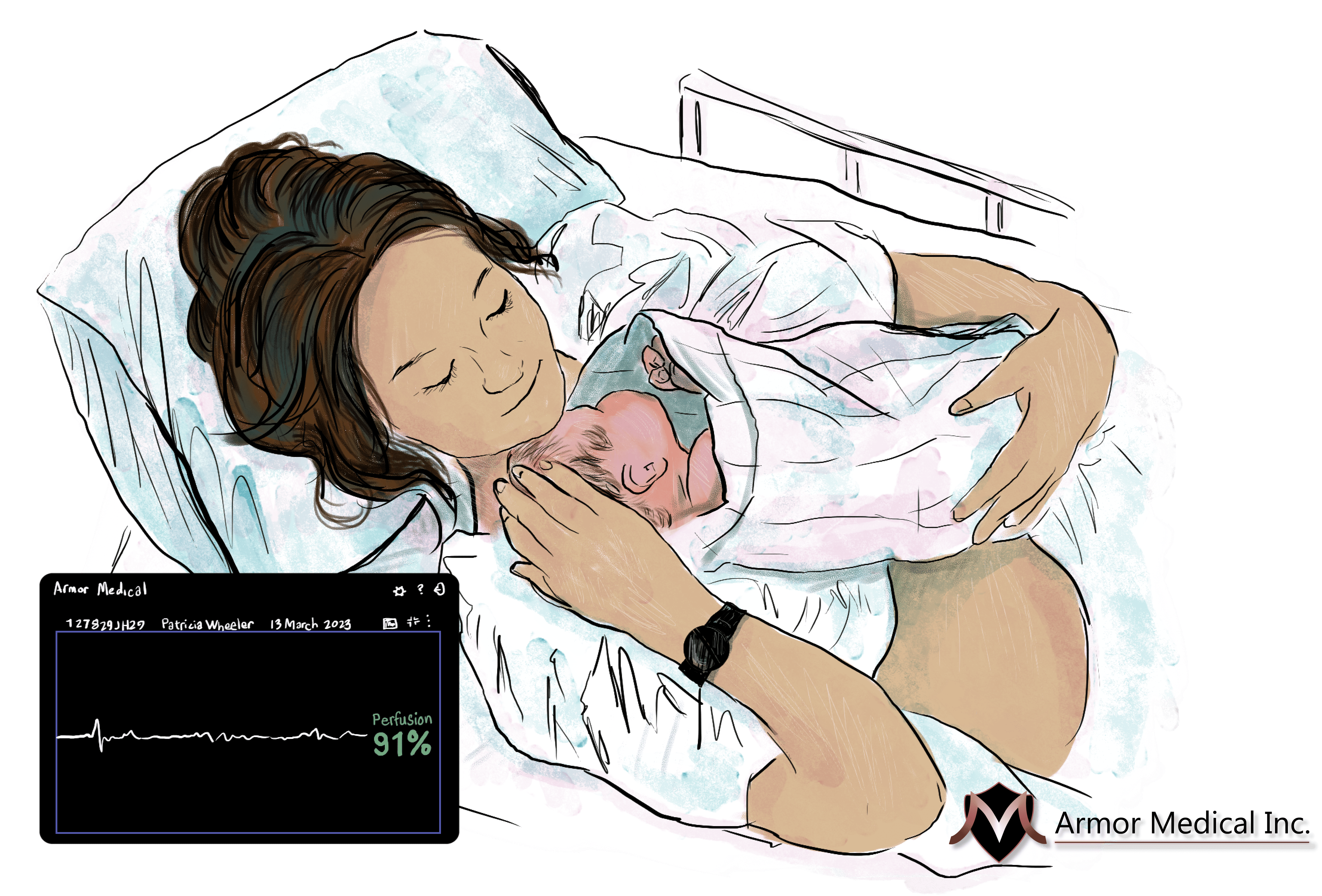
Currently, “providers don’t have tools to detect early stage hemorrhage,” said Kelsey Mayo, Co-founder and CEO of Armor Medical. “By the time we see blood, it’s too late.”
Last spring, the company was awarded $75,000 in funding from the National Institutes of Health’s Rapid Acceleration of Diagnostics Technology (RADx Tech) for Maternal Health Challenge. Armor recently developed its first commercially manufactured minimum viable product, and expects Maternal Armor to be available in 2025. The company is pursuing a breakthrough device designation through the FDA.
After that, Mayo would like Maternal Armor to become part of the monitoring that all birthing parents receive before, during and after delivery.
“Every mom deserves the chance to have this as the standard of care,” Mayo said.
Postpartum hemorrhage costs the U.S. more than $1.8 billion annually, so preventing it is not only an opportunity to save lives, but a massive untapped market, according to Mayo.
“It’s an extremely exciting time to be an innovator in this space,” she said.
Elidah
Their Value Prop: Non-invasive incontinence treatment that patients can use at home
Elidah is addressing a common but embarrassing problem: urinary incontinence, which impacts up to one-third of women. Most of those people learn to live with bladder leaks, since standard treatment options are invasive and costly surgeries, according to Elidah Founder and CEO Gloria Kolb.
Elidah offers a solution, with non-invasive treatments that patients can do at home, while they’re working and going about their daily activities. Elidah’s ELITONE® device provides low-frequency electric pulses to strengthen the pelvic floor. The device is FDA-cleared and more than 15,000 patients are using it for both stress incontinence and urge incontinence (also known as overactive bladder), Kolb said.

Although incontinence is a private problem, addressing it has widespread social impact, she noted. The cost of incontinence in the U.S. healthcare system exceeds $20 billion, with incontinence being the main reason people enter nursing homes, Kolb said. The condition is also linked to falls, depression and lack of physical activity.
Elidah recently closed its Series A round of funding, bringing the total investment to date to more than $4 million. The company is already profitable, and over the next 18 months, Kolb anticipates rapid growth, as Elidah breaks into foreign markets while continuing to grow its American user base.
“The market is so large that we have barely scratched the surface,” she said.
Mother of Fact
Their Value Prop: Saving lives by providing nutritional support to reduce pregnancy complications
Mother of Fact aims to save lives by improving nutrition for moms and babies. Of the 80% of maternal deaths that are preventable, one-third are directly linked to poor food access or nutrition status according to the National Institutes of Health.
Mother of Fact uses an integrated nutrition platform staffed by credentialed dietitians to offer continuous, high-risk nutrition care spanning from pre-pregnancy through a baby’s first year of life. Unlike other digital health players that often focus solely on baby feeding care, Mother of Fact provides comprehensive evidenced-based and high-touch nutrition support for mothers and babies, according to Founder and CEO Emily Sylvester, a registered nurse. Using the app can improve maternal health outcomes and diminish nutrition-related health disparities, including risk of life-threatening conditions like preeclampsia.
The app is already live in two states, backed by NIH funding. In the second quarter of 2024 it will launch with large health systems in two additional states, with plans to reach five states by the end of 2024, according to Sylvester. At that point, Mother of Fact will be serving 1,500 pregnant people each month, and conducting clinical trials on the impact of nutritional care. Sylvester plans to leverage the results of those trials to grow the app’s reach.
“After 18 months, this data will inform policy and value-based care endeavors allowing us to target regional Medicaid expansion,” Sylvester said.
The platform, she added, generates additional revenue through underutilized medical-nutrition therapy reimbursement codes, optimizing nursing staff time for higher-reimbursable tasks, and furnishing valuable social determinants of health data. The biggest value, however, is from the platform’s leadership team, composed of mothers and health care providers with intimate knowledge of the problem they’re hoping to address.
“We come from the communities and have worked in the hospital systems where we’re trying to ensure meaningful impact on these really underserved, vulnerable communities,” Sylvester said.
NovaGyn
Their Value Prop: Bioengineering better surgical treatments for incontinence
NovaGyn is also addressing incontinence, in this case by using biofabrication and tissue engineering to improve surgical treatments. The current surgical mesh used to treat stress urinary incontinence (SUI) can cause tissue erosion and other painful complications, said NovaGyn Founder and CEO Rebecca Thomson. NovaGyn’s technology harnesses the body’s natural healing power.

“NovaGyn’s tunable nanofibrous scaffold utilizes the body’s ability to create healthy connective tissue to build a permanent support for the urethra in women who suffer from SUI, eliminating the notorious side effects of synthetic mesh,” Thomson said.
The team—which includes three academic professors, two clinical experts, and two entrepreneurs—is currently optimizing the product through in vitro studies. While NovaGyn is still five years from the first human trials, 2024 will mark important milestones for the technology, as the company’s first Small Business Technology Transfer research grant is resubmitted.
“These mechanisms are great opportunities to raise capital and collaborate with subject matter experts,” Thomson said.
Surgical solutions for incontinence is a relatively uncontested industry, Thomson said. In addition, the market opportunity for NovaGyn’s technology expands well past addressing incontinence.
“Our product has the potential to make a bigger impact in other mesh-using markets such as other pelvic floor reconstructive, gastrointestinal, and hernia surgeries,” she said.





