ActiveStep: A Research Translation Success Story
Simbex is deeply rooted in the translation of research into practical, life-changing innovations. One of the company’s earliest projects, ActiveStep, embodies many of the core principles that have guided Simbex’s journey over the past 20+ years. Founders Rick Greenwald and Jeff Chu, both with strong research backgrounds in biomechanics and injury prevention/rehabilitation, found a way to combine their expertise into a novel medical device.
This project demanded a challenging blend of electromechanical design and sensor-driven innovation, areas where the Simbex team excelled. The technical prowess showcased in ActiveStep has been a consistent thread throughout the company’s history and remains central to our work today.
- An example of our sensor-based work can be seen in the Riddell Insite project
- Our continued expertise in the topic of fall prevention is demonstrated in the Silvertree Wearable solutions project
The primary purpose behind ActiveStep was to address a pressing issue within rehabilitative medicine: the high incidence of injurious falls among older adults. The underlying concept was that fall-avoidance can be trained like any skill, through repeated exposure and practice. This idea had been previously explored by researchers and clinicians who used various methods, including having patients walk along a walkway while suddenly introducing obstacles, unstable surfaces, and unexpected movements to mimic conditions that lead to falls. During these exercises, the patients would be protected from injury by using an overhead track with a safety harness or a therapist who would catch them if they fell.
However, these makeshift systems were far from ideal. They presented significant safety concerns, were cumbersome to set up, and often required considerable space. ActiveStep proposed a more elegant approach: a self-contained, controllable and replicable fall simulation system within the footprint of a common exercise equipment item – the treadmill.
To bring ActiveStep to life, Simbex teamed up with a leading fall researcher and biomechanist, Mark Grabiner. Dr. Grabiner played a crucial role in shaping the technical requirements of the ActiveStep product and piloting the developing technology in his lab. Even after product development, Dr. Grabiner continued to use Activestep in his research and both authored and co-authored more than a dozen peer reviewed publications related to ActiveStep.

Making The Most of SBIR Funding
In 2004, Simbex was awarded an SBIR phase I grant to explore the technical feasibility of bringing such a product to market. Initial pilot testing proved promising with a small study of 30 older adults. Building on this success, Simbex went on to secure an SBIR phase II grant in 2005.
Leveraging early, non-dilutive funding provided by SBIR grants was pivotal for Simbex in developing a complex but niche product such as ActiveStep. The ability to utilize grant funding to offset the expense of product development became another one of Simbex’s strengths throughout the years. Simbex would eventually go on to receive 15 SBIR phase I awards and 12 phase II awards, resulting in an impressive conversion rate of 80%. This track record of successfully obtaining funding and translating research into real life products established Simbex as experts in the product development and commercialization process.
This expertise did not go unnoticed. Both the NIH and FDA recognized Simbex’s capabilities and awarded the company three separate grants to run innovation centers called TREAT and NEPDC, which assisted academic and clinical innovators with commercialization efforts. Collaborating with external innovators through center grants propelled Simbex’s transformation from exclusively developing our own products to adopting a business model focused on strategic consulting and contract engineering for other companies’ projects, many of which are funded by SBIR/STTR grants.
ActiveStep – From Product Vision To Reality

By 2007, ActiveStep became a 510k exempt, class I medical device. In its final form, it consisted of a rugged treadmill with a pressure sensor under the belt, a safety arch with a harness system, and an external computer that controlled the system and provided data analytics. To a casual observer, it might appear as a common treadmill with a bodyweight support system. However, ActiveStep’s unique capabilities made it much more than a standard treadmill.
Functionally, the most important feature of the ActiveStep device was the powerful motor that had the ability to rapidly accelerate, decelerate and reverse the treadmill belt. The design of this feature was one of the most challenging aspects of the device, since one product requirement was that ActiveStep could be plugged into a normal 120v outlet. This would enable ActiveStep to connect anywhere without requiring an electrician to install a 220v plug.
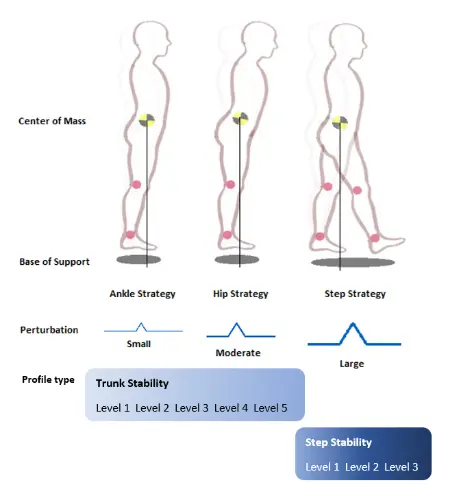
ActiveStep’s unique ability to generate sudden movement of the ground underfoot caused the subject’s base of support to shift forward (initiating a slip that would cause them to fall backwards) or backwards (imitating a trip that would cause them to fall forwards). ActiveStep could initiate these slip and trip movements when the patient was in a standing position or when they were walking.
ActiveStep featured five levels of perturbation intensity, allowing patients to start with mild movements and gradually work their way up to more challenging levels. This gradual progression was essential because patients at risk of falling, especially those who had fallen before, often had a significant fear of falling again. Therapists could use the lower settings to orient patients to the exercise, helping them become comfortable with the treatment before moving on to higher-intensity perturbations.

As patients progressed through the levels of perturbation, they learned to use various techniques of countering the fall, allowing them to regain their balance. At lower levels of perturbation, patients could often recover by swaying their bodies using primarily ankle movements to regain an upright posture. For more intense perturbations, or if the patient’s leg muscles were weaker, they might need to use larger trunk and upper body movements, hinging at the hips and flinging their arms out for counterbalance. At the highest levels of perturbation, the only way for a patient to recover and avoid a fall was to take a quick step in the direction of the fall. This recovery step was crucial for rapidly moving the base of support under the center of gravity, and it was the primary skill that ActiveStep aimed to train.
While fall recovery responses occur reflexively in healthy patients, older or weaker patients cannot react powerfully enough to prevent a fall. ActiveStep exploited an interesting phenomenon: reaction steps and other responsive movements can be improved with practice, even in individuals who are prone to falling. Just like with any other movement, this learning effect is stronger with more repetitions. However, the learning curve appeared to be very steep with ActiveStep. Dr. Grabiner’s research found that even a single 15-repetition session on ActiveStep significantly improved the recovery motions of subjects with chronic stroke.
The other specially-designed features of ActiveStep also contributed to its usability and effectiveness. The custom fabricated arch and harness system provided a safe environment that prevented the subjects from falling all the way during practice. The pressure-sensing mat under the treadmill belt allowed for gait analysis and provided data about stride length, width, speed, pressure, symmetry, and most importantly, reaction step time. These measurements could be used to provide gait training and coaching for the patient, and they were also used behind the scenes to time the belt perturbations to occur at the time in the gait cycle when they would be the most effective for training. Some basic gait data could be viewed within the user interface, and if ActiveStep was being used in a research environment, the raw data could be exported for further analysis. The system also stored patient records, allowing patients and clinicians to track progress across multiple training sessions.
A staple In Falls Research
ActiveStep sales and manufacturing was kept in-house at Simbex. This allowed us to “right size” the process to fit demand. The predominant customers of the device were researchers who were interested in studying the biomechanics of falling. ActiveStep had been designed with the requirements of researchers in mind, and its adoption by biomechanics labs throughout the country demonstrated its usefulness to this type of customer.
ActiveStep could be integrated into their gait labs and combined with EMG and camera-based motion capture systems to allow for advanced kinematic and kinetic research. One of the most valuable aspects of the ActiveStep system for this customer group was the ability to control the intensity of the perturbation. This allowed for standardization across study subjects and repeatability between sessions, opening the door to a wider variety of study protocols compared to the alternative methods of inducing falls.
Research Laboratories with ActiveStep include:
- University of Illinois at Chicago
- The Mayo Clinic
- Central Michigan University
- University of Maryland, Baltimore
- University of North Carolina at Greensboro
- University of Texas at El Paso
- Georgia State University
- University of Delaware
- University of the Sciences in Philadelphia

The original product vision for ActiveStep was for it to be adopted as standard equipment in physical therapy practices. In actuality, the use of ActiveStep in physical therapy practices was limited to a specific type of practice: those that saw a large volume of older adults, and those that used primarily cash-pay or value-based payment models. The primary reason for this was that ActiveStep didn’t fit neatly into the standard fee-for-service reimbursement system. There is no additional payment mechanism for using advanced equipment like ActiveStep. Therapists typically billed for therapeutic exercise, neuromuscular re-education, or gait training when they used ActiveStep during an appointment. All of these services could be billed using more affordable equipment such as exercise balls and stretch therapy bands. This lack of additional reimbursement basically disincentivized the use of advanced rehabilitation technology. This is an issue that remains a challenge in the physical therapy space, and it is one that many Simbex customers encounter.
Despite these barriers, ActiveStep did still make its way into some clinics, primarily those with cash-pay or value-based payment models. In these models, the patient’s outcomes is the ultimate metric for reimbursement and access to an effective clinical tool can be a key differentiator from the competition. These practices found that integrating ActiveStep into their standard of care could reduce injury rates and improve quality-of-life metrics, especially in older adults.
A large-scale pragmatic clinical trial published in 2020 compared a standard treatment protocol to treatment that included ActiveStep training. The 8-center study included more than 500 patients over age 65 and found that adding ActiveStep to usual physical therapy resulted in a reduction of injurious falls up to three months after treatment. The results of this study were compelling and suggested that ActiveStep could play a significant role in reducing fall-related injuries among older adults in clinical settings.
When marketing the product, Simbex considered several “outside the box” options for ActiveStep. For example, an ActiveStep unit was installed in a mobile therapy unit that traveled to various VA locations on a semi truck. Given that ActiveStep delivers a powerful training effect in a short period, one idea was to approach it as an annual preventive measure or inoculation against falls. With this thinking, patients could return for a “booster” of treatment periodically. Patients that are highly motivated to age in place and stay active without the fear of falling might be inclined to purchase a membership, akin to a gym membership, granting them access to ActiveStep whenever they wish to practice their fall recovery skills.
In 2020, Simbex announced a partnership with Biodex to integrate ActiveStep technology into their GT3 treadmill. This could allow a wider audience to utilize a simplified version of perturbation training at a lower price-point. The hope is that this increases adoption and access to perturbation training in clinical settings.

Lessons learned and transferable knowledge
The Simbex team became familiar with the process of product development from the inside out while developing its early products like ActiveStep. The insights gained during that process continue to influence the development plans we execute with our clients today. Many of our current key focus areas emerged from the challenges we faced during the commercialization of ActiveStep, particularly the slower-than-expected adoption of the product in its originally intended market: physical therapy clinics.
We accidentally built a research tool when we had originally intended ActiveStep to be a rehabilitation platform. In retrospect, we can understand why this happened. A key factor was that we based our product requirements on the use case of a researcher. The cost of the product, the extended feature set, and the onboard analytics all proved to be more appropriate for the laboratory environment than the clinical environment. We had failed to consider the typical clinical workflow, especially the fast-paced therapy appointments, that make set-up and take-down times critical. We had also designed a feature-rich and complex UI that had a steep learning curve and displayed more than the bare minimum outcomes data, including nested menus and operations that required multiple mouse clicks.
As a research tool, ActiveStep was actually a huge success, leading to important discoveries about the nature of falls and fall reaction movements. Throughout the years, ActiveStep was used as study equipment in more than 200 peer-reviewed publications
This indirectly will have an impact on treatment protocols and will eventually filter down into daily practice. However, commercially speaking, tapping into the clinical market would likely have led to the sale of many more units, since there are a significantly larger number of physical therapy practices than biomechanics labs.
The importance of phase 0
One of the key lessons learned from ActiveStep was the importance of ensuring that product requirements are in sync with the needs of the target market. This experience has influenced the strategies in which Simbex currently employs to approach new projects. We now emphasize a “Phase 0” approach to product development, which involves gathering input from a wide range of stakeholders at the outset of a project. We create customer journey maps to make sure that we fully empathize with their point of view and understand their needs. We encourage extensive user testing at each stage of development, using a design-thinking approach that iterates based on feedback.
Another strategy that we integrate into our process at Simbex is the evaluation of the need and opportunity within the market. This enables us to target the customers with the best fit for the product and estimate the overall sales volume, informing the scaling of our R&D efforts. We encourage the innovators that we work with to develop a realistic idea of who the early adopters of their technology will be so they can leverage these early successes and build toward greater penetration of the potential market.
A part of this early market assessment is planning for reimbursement and regulatory pathways. We help guide commercialization efforts to avoid foreseeable barriers to adoption. We also evaluate trends and try to anticipate changes in the landscape ahead of time. For example, the recent pandemic made home therapy a popular option. Smaller, wearable, and portable rehabilitation tools are increasingly popular as physical therapy moves outside the four walls of the clinic. In this climate, the sale of expensive capital equipment is more challenging than ever. Patient monitoring equipment is growing in popularity, and the miniaturization of technical components is making it possible to build small systems that can be used at home and in the community.
Another component of the analysis that we perform during Phase 0 is concentrated on understanding what the barrier to the sales process might be. Equipment that is expensive, bulky, or requires extensive practitioner education in order to use it will most likely encounter adoption resistance. We emphasize the importance of conducting these analyses early in the development process because their results can significantly impact technical development. Planning for this in advance enables us to integrate features that make it easier to use and sell.
The process of manufacturing medical devices like ActiveStep in-house has shaped the product development methods at Simbex. As an FDA-registered medical device, ActiveStep manufacturing was required to follow Good Manufacturing Practices, which meant maintaining a compliant Quality Management System (QMS). Implementation of the QMS shaped the standard operating procedures of the development process throughout the company.
Simbex was also responsible for maintaining and reconditioning the ActiveStep product, which gave us the unique opportunity to revisit our own engineering work on a regular basis.
As the years elapsed, we revised the design of ActiveStep a few times in order to accommodate updated components and to address common failure modes. One area of expertise that has emerged from this process is our team’s proficiency in reworking and modernizing legacy medical devices. This is a skill set that not every engineering house has, and we lean heavily on our experienced mechanical and electrical engineering team to analyze the current situation and suggest workable retrofit solutions. Because we have a talented and integrated multidisciplinary team, our firmware, software, and algorithm engineers can also contribute their suggestions to the hardware design to allow for integration of sensors and other “smart” componentry. This is at the heart of what we do and the roots of it can be seen in the heritage of ActiveStep.
Continuing the Mission: Collaborating with Visionary Innovators
The ActiveStep story is one example of the many medical products that Simbex has created. We have experienced the process inside and out, from napkin sketch to regulatory filing to manufacturing. This story contains just some of the successes and failures that we have experienced and contributed to the level of expertise that we have gained throughout the last ~25 years of medical device design and development.
Our team enjoys nothing more than collaborating with visionary innovators. If your team is working on a product similar to ActiveStep, Simbex would be excited to collaborate with you to avoid common pitfalls on the path to market and build something truly exceptional together. Our mission is to design and develop medical devices and consumer health products that make an impactful difference in the world. We believe that the best way to accomplish this is by mixing our experienced team with your innovative product ideas.
Working with Simbex can take many different forms. A few key subject-matter experts from our team can integrate into an existing product development team and provide technical strength in a discipline that may be lacking from that team, or even help to support an over-committed workforce. In other cases, Simbex can overhaul a portion of a larger project or even start from scratch to see a project through in its entirety.
We enjoy the variety in what we do, for we recognize that no two projects are the same – that is what keeps us interested! Our work spans a wide range of project durations, from short-term prototyping tasks to decades-long partnerships where we serve as the fully integrated development team for larger companies.
If something in this case study resonates with your own product development journey, don’t hesitate to reach out! We offer free business development consultations to explore whether our team could be a good fit for your project.



