Advancing Sleep Disorder Diagnosis with Enhanced Performance Sensing Technology
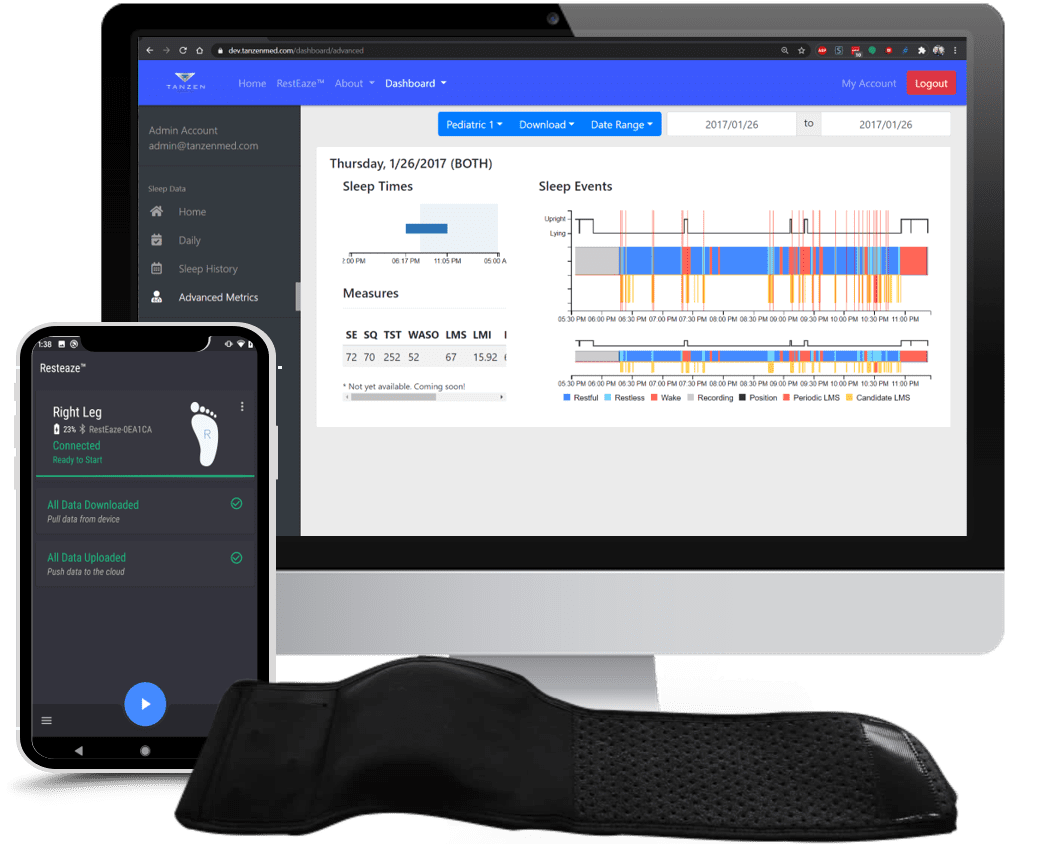
Simbex partnered with Tanzen Medical to develop a 501(k)-submission-ready prototype wearable, RestEaze, the first ambulatory, remote-monitoring device designed to aid in sleep disorder diagnosis including Restless Legs Syndrome (RLS), Periodic Limb Movements of Sleep (PLMS, insomnia), and Attention-Deficit/Hyperactivity Disorder (ADHD).
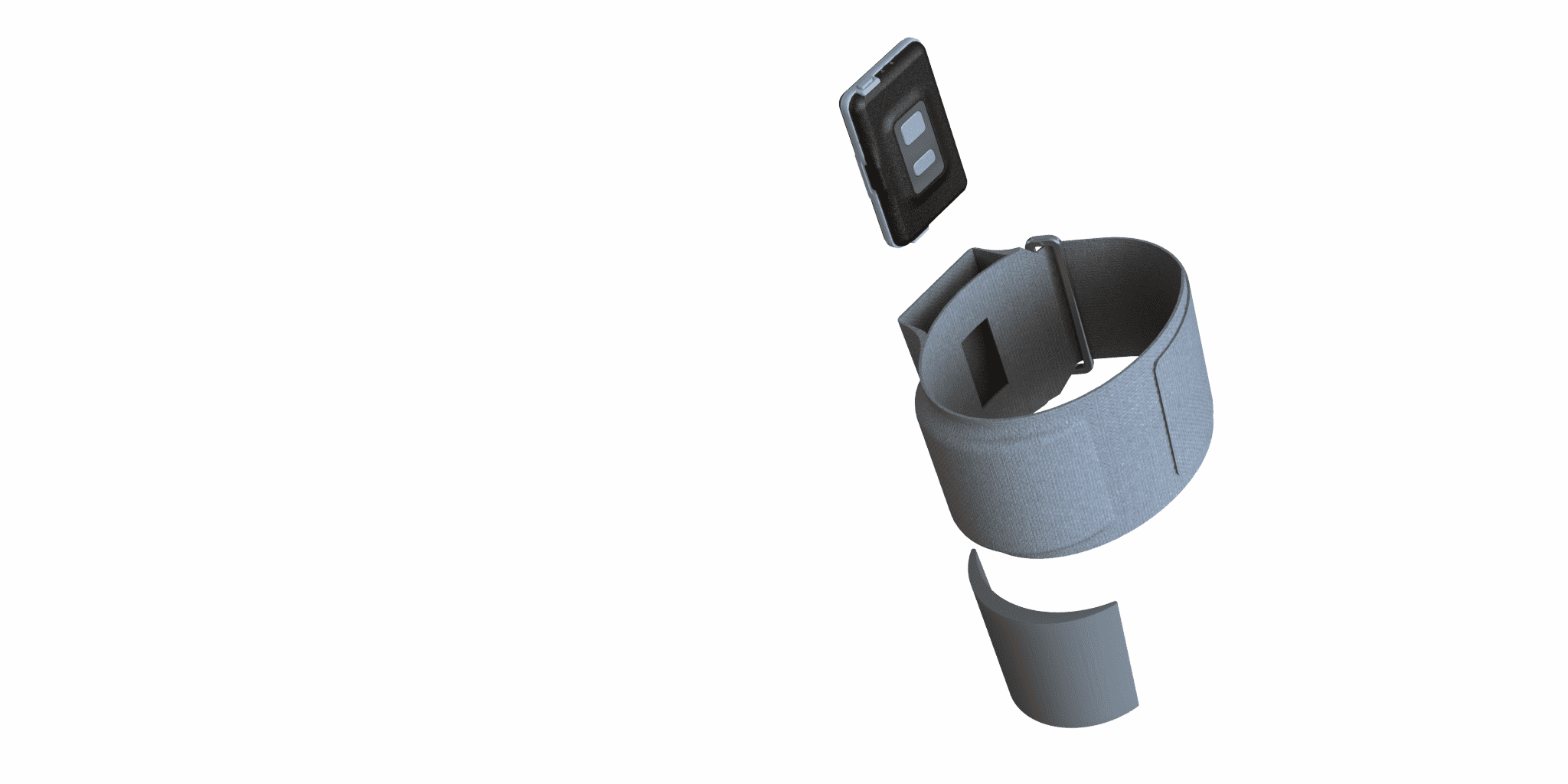
Tanzen approached Simbex with a prototype that included an in-house developed PCB, a very basic 3D-printed housing, and a preliminary strap system for holding the device on the leg. This original housing and electronics were meticulously refined, and a new strap was developed, transforming this preliminary concept into a sophisticated, market-ready product.
After a successful first engagement, Simbex is now developing an edge-processing algorithm for Tanzen’s next-generation device.
Challenge: Innovating Sleep Performance Sensing with RestEaze
The Need for Innovative Solutions in Sleep Performance Sensing
The quest for improving sleep quality and diagnosing sleep-related disorders has led to a significant interest in advanced monitoring technologies. Periodic Limb Movements during Sleep (PLMS) serve as a crucial biomarker for sleep movement disorders, signifying conditions such as low brain iron in children and potentially correlating with various other medical conditions in terms of presence and severity. Despite its importance, the current gold standard for PLMS monitoring—Polysomnography (PSG)—is laden with challenges. PSG’s invasive nature, requirement for numerous sensors, and the confinement to a laboratory setting not only make it an expensive option but also potentially discomforting for patients, leading to data that may not accurately represent their typical sleep behavior.
Tanzen approached Simbex with a Prototype RestEaze Device and Many Technical and User Experience Challenges:
- Miniaturization: Developing a device capable of accurate sleep monitoring while ensuring it remains unobtrusive presents a considerable technical challenge. The target thickness of 8-10 mm for market readiness necessitates innovative approaches in the miniaturization of components without compromising functionality.
- Prototype Refinement: Transforming an initial prototype, which may be rudimentary or “crude,” into a polished, market-ready product demands a comprehensive overhaul—encompassing design, engineering, and user interface improvements.
- User-Friendly Design: Ensuring ease of use is paramount. The device must be intuitive for the user, facilitating seamless integration into their nightly routine without requiring extensive setup or technical knowledge. The strap must also be designed to ensure that the sensor is positioned appropriately every time.
RestEaze: The Vision
RestEaze represents a groundbreaking approach to sleep performance sensing. By focusing on PLMS, RestEaze aims to offer a non-invasive, comfortable alternative to traditional PSG studies, enabling long-term, home-based monitoring. This innovative device employs machine learning algorithms trained on clinical data, ensuring its ability to classify leg movements during sleep with accuracy comparable to PSG outputs.
Simbex is an ideal partner for any wearable product because of our 20+ year history in biomechanics, deep expertise in algorithms and wearable sensors, and human factors sensibility.
Mechanical & Electrical Design/Engineering Approach
Simbex employs a systems integration viewpoint that emphasizes the importance of functional requirements and the product lifecycle. This approach ensures that RestEaze is designed not just for user needs but also considers seamless integration with external systems for manufacturing, distribution, packaging, and maintenance, addressing the entire ecosystem surrounding the product.
- Key Technologies: The development process incorporates advanced technologies such as 3D Modeling and Simulation, which is critical for visualizing and refining the design of the device. Ultra-Low Power Digital Circuits are utilized to ensure that RestEaze operates efficiently, conserving battery life while maintaining performance. Sensor Interfaces and Signal Conditioning are fundamental in accurately capturing and processing physiological data. Additionally, compliance with IEC 60601-1 for Medical Systems ensures that RestEaze meets international safety and performance requirements for medical electrical equipment.
Design & Human Factors
Incorporating user feedback is a cornerstone of Simbex’s design philosophy. This user-centric approach ensures that RestEaze is not only effective in its function, but that it also exceeds user expectations in terms of ease of use and comfort.
- User Experience Design: Understanding and implementing the user’s product requirements from the outset and continuously refining them based on feedback. In this case, understanding how the patient will apply the product and how the product needs to be designed so the sensor location is always correct.
- Product Verification & Validation: Rigorous testing methodologies are applied to ensure that every aspect of RestEaze meets the intended design specifications and user needs.
- Graphic Design: The visual aspect of the user interface is crafted to be intuitive, enhancing the overall user experience and facilitating ease of use.
Software & Web Development
Simbex adopts an agile approach to software development, ensuring flexibility and responsiveness to changing requirements throughout the development process. This approach is instrumental in developing embedded firmware and cloud-based solutions that form the backbone of RestEaze’s functionality.
- Embedded Firmware: Critical for the real-time processing and analysis of physiological data captured by RestEaze.
- Cloud-Based Solutions: Enable the secure storage, processing, and analysis of large volumes of data, providing valuable insights into sleep patterns and disorders.
- Verification/Validation: A focus on rigorous testing ensures that the software components of RestEaze function correctly, are secure, and meet all regulatory requirements.



