Transforming Ideas into Marketable Solutions: Simbex’s NIH-Funded Center for Translation of Rehabilitation Engineering Advances and Technology (TREAT)

Overview
From 2010-2020, Simbex was the coordinating center and heartbeat of the NIH-funded Center for Translation of Rehabilitation Engineering Advances and Technology (TREAT). During this time, TREAT educated and assisted researchers, clinicians, bioengineers, and medical rehabilitation and assistive technology inventors in the process and actual translation and commercialization of rehabilitation devices and technologies. Funded through NIH’s National Center for Medical Rehabilitation Research (NCMRR) P2C Medical Rehabilitation Research Resource Network (MR3), and in partnership with The Dartmouth Institute for Health Policy and Clinical Practice, the Thayer School of Engineering at Dartmouth College, and the Department of Physical Therapy in the School of Health and Rehabilitative Sciences at the University of Pittsburgh, the TREAT consortium’s overall goal was to provide comprehensive technology translation training for innovators.
Simbex took the lessons learned from our involvement with TREAT and other federally funded accelerator programs, along with our own product development experiences and industry best practices, to now apply these methods to provide both product development and commercialization strategy services to our clients. We have provided commercialization assistance to more than 500 individuals and companies since we began offering strategy services over 14 years ago. Specifically, we work with teams to focus on the broad spectrum of activities that are required to take a concept from the lab to the market, taking the approach of rapidly de-risking the commercialization approach. We use a “high-touch” approach where we engage with our clients through a diverse team of business development experts, clinicians, engineers, scientists, regulatory and reimbursement experts, and more in a focused effort to overcome existing barriers to commercialization.
At a Glance
Activities
- Group education- cohorts and online modules
- Individual Education and advising – TREAT Methodology
- Project Funding – grant application/review/award process
- TREAT Fellows – also short term internships for RESNA student award winners
Accomplishments
- Metrics – we advised a lot of people, reviewed a lot of ideas
- Highlight a few successful projects (Biomotum, Healthy Design, MedRhythms, Limbix (now Big Health, The Overcomer, VERVE from Oculomotor Technologies, Capti Voice, Zibrio))
- Transition to Commercialization Services
Background
Building on the Foundation of TREAT
New devices are developed every day to treat the health and well-being of individuals with disabilities, including neurological and neuromuscular disorders, as well as those rehabilitating from injury, but many of these important innovations never get to market to reach the people who can benefit from them the most. A well-known problem, the so-called “Valleys of Death” exist in the translation of novel technologies from research to clinical use, and relate to the inability, both financially and institutionally, to cross chasms from idea to product, confounding efforts to translate important research into clinically useful paradigms. Co-PIs Richard Greenwald from Simbex and Jon Lurie from the Dartmouth Institute set the intention to provide education and assistance to help innovators avoid the traps that can derail the medical device translation process (Figure 1).
Medical device development begins as an idea in the mind of a researcher, clinician, or engineer, whose background might not include specific training or experience in commercialization. Too often, there is a scarcity of resources to educate and support the innovator through the complexities of development and execution. Leaving this process in the hands of university and hospital technology transfer offices and small business accelerator programs (e.g. I-Corps, Larta, SBIR, AngelList, SBA, etc.) may result in general entrepreneurial guidance that is not adapted for the unique needs of medical device development. If the innovator does find help from specialized commercialization centers and programs that are oriented toward medical device development, it is common for those resources to focus only on singular aspects of commercialization, such as IP, regulatory matters, or product design. The result of this fragmented approach can be confusion, discouragement, or “paralysis by analysis”, with the outcome being expense, delay, or failure to translate the device to market.
Figure 1:
TREAT, funded through the NIH from 2010 to 2020, was a collaborative consortium providing education, assistance and resources to rehabilitation device developers in partnership with The Dartmouth Institute for Health Policy and Clinical Practice, Simbex, the Thayer School of Engineering at Dartmouth, and the Department of Physical Therapy in the School of Health and Rehabilitative Sciences at the University of Pittsburgh.
TREAT operated on the understanding that there is a world of difference between reading about the aspects of technology transfer (e.g. patents, licensing, royalties, ethics, grant-writing) and actually experiencing and practicing the process of product development and strategic product launch/marketing.
Commercializing Medical Devices
Medical devices face many fundamental challenges with the development of any consumer product, including the requirements that the device must solve a critical need for a specific type of application and be properly marketed to those who will benefit from it. However, in addition to these general commercialization considerations, the clinically critical application of medical devices necessitates attention to topics such as planning for the regulatory process, understanding reimbursement, and developing strong clinical rationale, including bioethical considerations and clinical trials to determine comparative effectiveness.
Drawing on Past Success
The commercialization path for medical devices can take many forms. The complications inherent in the translational process necessitate an agile and comprehensive approach to diligence and development. Through TREAT, Simbex was involved with hundreds of product concepts and thus became adept at delivering highly specialized assistance in response to the needs of the innovator, the resources available, and the requirements of the project.
Zibrio is an example of a product we helped early in their development that is making an impact today:
Zibrio: A quick, low-cost diagnostic tool, the Zibrio scale assesses balance and fall risk for home and hospital. Zibrio offers the ability for balance and fall risk to become part of a general wellness check alongside height, weight, and blood pressure. Former NASA scientist Dr. Katharine Forth sought help from TREAT to better understand the product’s regulatory pathway and manufacturing quality controls. She also learned the value of in-depth customer interviews in developing a go-to-market strategy and product requirements. The Zibrio team is presenting widely at conferences and pitch competitions and have completed a successful crowdfunding campaign.
Other products we helped early in their development that are also making an impact:
- Zibrio: https://www.zibrio.com/
- Biomotum: https://www.biomotum.com/
- Healthy Design: https://www.hdmedical.org/
- MedRhythms: http://medrhythms.com/
- Limbix (now Big Health): https://www.bighealth.com/
- The Overcomer: https://www.inclusivity-fitness.com/
- VERVE from Oculomotor Technologies: https://www.oculomotortechnologies.com/
- Capti Voice: https://www.captivoice.com/capti-site/
The TREAT Commercialization Methodology
Successful commercialization is dependent on a wide variety of factors across several domains, including business, clinical, technical, financial, and, in the medical device space, regulatory and reimbursement. Each of these domains presents unique risks that require sound judgment and properly timed assistance. TREAT developed its Commercialization Methodology as a high-level means of communicating the many steps required to successfully bring a medical device to market.
The TREAT Commercialization Methodology was intended to be used as a roadmap of the appropriate tasks and activities which must be completed in each stage of the product development pathway. Many product development process maps are available, but most focus on the technical aspects and ignore the clinical and business considerations. Very few product development resources are available that encompass the unique requirements of medical device translation. The TREAT Commercialization Methodology was used to effectively coach innovators to complete due diligence on their ideas, considering the clinical, ethical, regulatory, reimbursement, and business repercussions from the ideation stage of the project (Figure 2).
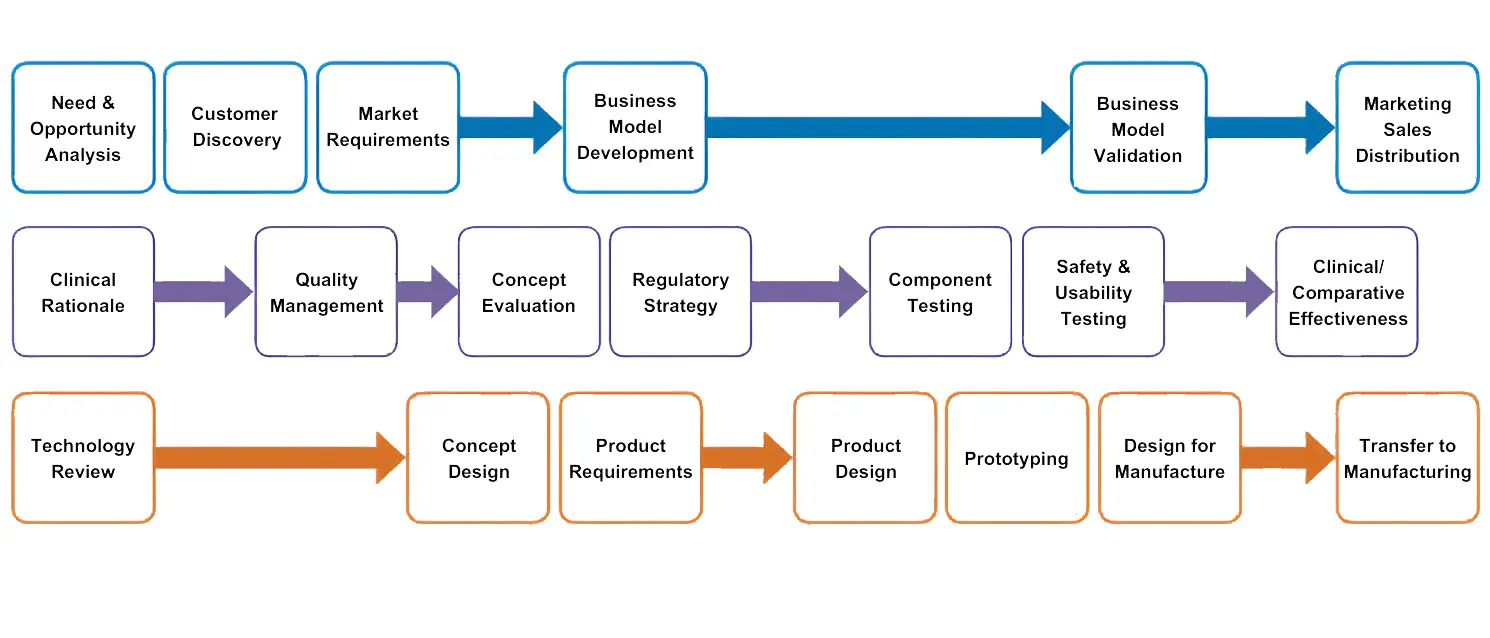
Figure 2:
The TREAT Commercialization Methodology facilitates a holistic approach to medical device development. The three rows of the diagram represent the three main fields of activities necessary to commercialization: business development (top row), clinical/product evaluation (middle row), and technical development (bottom row).
A common mistake made by first-time innovators is to move forward with costly technical design and prototyping without first identifying the primary economic buyer and design requirements that will satisfy key stakeholder needs. This scenario results in business and technology development being out of phase, putting the project at risk of falling into the “Valley of Death”. The Methodology considers each of three interrelated business, clinical and technical domains throughout the commercialization process of any medical or consumer health device. Each of the three focus areas contributes equally to project success. The Commercialization Methodology served as a guide for the innovator, encouraging equal focus and balancing of business, clinical, and technical development status as the project moved forward. Following the sequential activities outlined in the Commercialization Methodology helps to reduce errors by encouraging early-stage due diligence (Table 1).
TREAT Pilot Projects
TREAT’s flagship program was its Pilot Project awards, providing technology assessment, grant funding, and in kind mentorship via three grant cycles per year. Through program promotion and outreach to the rehabilitation community, TREAT solicited project ideas from innovators in the areas of rehabilitation and assistive technology. Product concepts came from small businesses, academic researchers, clinicians such as physical therapists and prosthetists, and people with disabilities or their caregivers who had a problem they needed solved and had devised a way to solve it. Through a two-tiered process, applications were reviewed based on demonstrated rehabilitation needs, innovation of the device concept, commercialization potential, and potential value added by TREAT assistance. All applicants received feedback and were often directed to relevant educational materials or were invited to apply to an educational program. Two to four applicants per cycle received awards of seed funding and/or in kind services. The newly awarded TREAT clients worked closely with TREAT mentors to develop a personalized commercialization assistance plan focusing on specific tasks, deliverables, and a timeline designed to meet their project goals and move them toward a commercially viable product. In the five years of the TREAT 2.0 grant (2016-2020), TREAT provided assessment and feedback to 569 device concepts (abstracts) and 281 Pilot Project Grant Applications and awarded 42 Pilot Project Grants of funding ($562,720 total) and in kind services.
The Educational Focus of TREAT
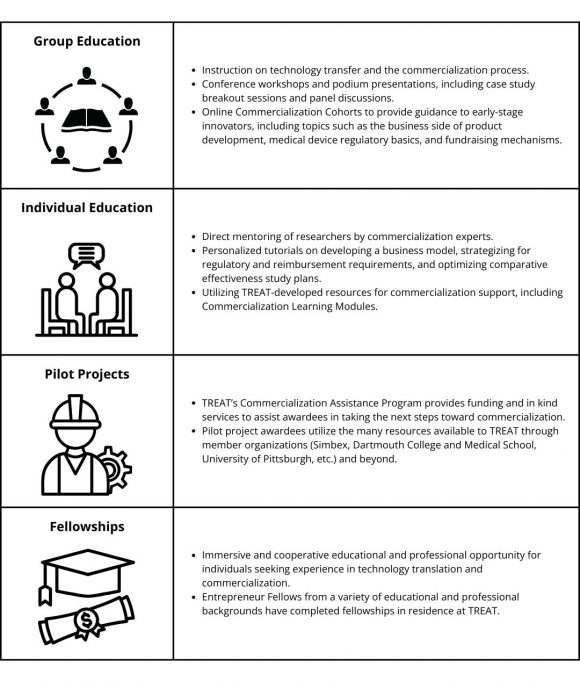
TREAT provided a critically lacking infrastructure resource for researchers that had device concepts in mind or projects ready for translation to the clinical environment and were ready to learn about the process of product ideation, evaluation, execution, and technology transfer. TREAT filled the gap between the efforts made by universities and government to explain and de-mystify the tech transfer process and the market process through educational opportunities, pilot projects, and fellowships (Table 2).
TREAT leadership developed a variety of educational programs to reach as large an audience as possible, from self-guided online learning modules based on the Commercialization Methodology to intensive personal mentoring of Pilot Project awardees over several months. As an intermediate option, TREAT developed an online Commercialization Cohort model, providing a structured series of webinars and assignments that included personal interaction with TREAT mentors and networking opportunities.
Table 2: TREAT Program Components
Click to Expand
TREAT Fellows and RESNA Interns
The TREAT Fellows program supported eight fellows-in-residence who were selected through a competitive application and review process and participated full-time in TREAT programs in staggered six-month intervals. The pool of fellows consisted of a wide range of backgrounds (clinical, technical, and business) and experience (graduate, post-doctoral, and industry professionals).
TREAT maintained an active partnership with the Rehabilitation Engineering and Assistive Technology Society of North America (RESNA) throughout the ten years of its program. Each year TREAT provided review support and sponsorship of their Student Design Competition, including the special TREAT award for “Technology Most Likely to Become Commercially Available.” Winners of the TREAT award received funding and in kind services similar to Pilot Project awardees and were invited to Simbex for short, in-person internships to further develop their technology and receive commercialization mentorship.
Pilot Projects
In total, 42 Pilot Projects have been awarded seed funding and in kind service hours to accelerate development of their rehabilitation technologies:
- Exercise Restraint: A restraint device that provides a safe means of early mobilization to prevent muscle atrophy while deterring self-extubation for patients in the Intensive Care Unit. Awardee: Marie Pavini, MD FCCP; Rutland Regional Medical Center
- Pediatric Evaluation of Disability Inventory – Patient Reported Outcome (PEDI-PRO): PEDI-PRO is the first PRO software designed to meet the needs of the broad population of youth with developmental disabilities. The software incorporates a user interface and workflow to improve validity of the self-reported measure. Awardee: Jessica Kramer, Ph.D.; Boston University
- Easy Step Soft AFO Brace: Easy Step is a soft brace / ankle-foot orthosis that integrates into a wide array of footwear to provide walking assistance for foot drop. Awardee: Suzanne Malinowski; New England Foot Drop Center
- Build-A-Stretcher (BAS): BAS provides a means of building a stretcher around a patient, raising the patient off the mattress, and laterally wheeling a patient off the bed, over a wheeled base, and onto a gurney. Awardee: James Denosky, Nolifting Mobility
- ViTAL: Classroom assistive technology that translates visual content displays into content that can be seen and heard through sounds and felt through vibrations on touchscreens. Awardee: Jenna Gorlewicz, Ph.D. Saint Louis University and ViTAL
- LiteRun: Lite Run helps patients regain mobility using air pressure inside a specially-designed suit. The air pressure inside Lite Run’s suit lifts a patient off of the ground to unweight some of a patient’s body weight onto Lite Run’s walker. Awardee: Doug Johnson, LiteRun Inc.
- UNYQ Scoliosis Brace: A scoliosis treatment based on proven bracing methodologies and a patient-centric philosophy. Awardee: Evy Wilkins, UNYQ
- Lite Run Pediatric Gait Trainer: An innovative device in the treatment of children with gait and balance difficulties. The system consists of a sophisticated Gait Trainer and specially designed “space suit” pants, which use air pressure to support up to 50 percent of a child’s body weight. Awardee: Douglas Johnson, Lite Run, Inc.
- PlayGait: A pediatric exoskeleton designed to increase the quality and quantity of a child’s walking while also being comfortable, discreet, and affordable. Awardee: Katherine Steele, PhD, University of Washington
- Electrical Stimulation-Assisted Video Game Hand Therapy for Cerebral Palsy: A custom electrical stimulator assists weak hand opening in proportion to the opening of the stronger hand. Awardee: Michael Fu, PhD, Case Western Reserve University
- Aipoly: Aipoly helps people who are blind and visually impaired quickly identify objects using affordable cutting-edge technology. Awardee: Alberto Rizzoli; Aipoly
- Pow!r Mount: Pow!r Mount is a modular motorized mounting system that attaches to a wheelchair, bed, or table and is used to position devices, including a speech device, camera, laptop, or tray.
- Awardee: Dianne Goodwin, BlueSky Designs
- Personal Wheelchair Platform: The Personal Wheelchair Platform is an innovative and cost-effective tool for evaluation and training of wheelchair propulsion in order to improve efficiency and prevent orthopedic injury in manual wheelchair users. Awardee: Jacob Rammer, Engability Inc.
- MedRhythms Stride: MedRhythms Stride provides people who suffer from neurologic injury or disease access to the highest quality of walking rehabilitation through an advanced neurologic music therapy technique combined with wireless sensing and deep learning. Awardee: Owen McCarthy, MedRhythms Inc.
- CaptiVoice: Educational text-to-speech app for students with print disabilities. Awardee: Yevgen Borodin, Ph.D. Charmtech Labs, LLC
- Smart Hand: Virtual reality game compares affected and unaffected limb for stroke rehabilitation of upper limb function. Awardee: Preeti Raghavan, M.D., NYU Rusk Rehabilitation Center
- Reflex Training Device and Protocol: Hardware and software nerve stimulation to modify nervous system pathways to improve locomotion. Awardee: Diane Borghoff, M.S., CT(ASCP) Health Research, Inc.
- Kickstart: A neurorehabilitation device designed to accelerate functional improvement and motor recovery following a stroke, spinal cord injury, multiple sclerosis, or other neurological condition. Awardee: Brian Glaister, MS, Cadence Biomedical
- SpeechMatch: An easy and fun game-like tool that provides social skills speech training for people with autism spectrum disorder. SpeechMatch enables language acquisition and improvement to continue when speech therapists are not present. Awardee: Robert Taub, DMA and Lawrence Welkowitz, PhD, Tembor, LLC
- Gait Buddy: The Gait Buddy device attaches a wheelchair to a walker, enabling a single therapist to provide hands-on gait training in a manner safe for both therapist and patient. Awardee: Cassi Petsch, PhD, Petsch Therapeutic Innovations, LLC
- Frogpad: An easy-to-learn fully functional portable keyboard that allows for every function of a standard Qwerty keyboard to be accomplished with one hand. Awardee: Linda Marroquin, Frogpad, Inc.
- Zibrio: A scale that assesses balance and fall risk for home & hospital. A quick, low-cost diagnostic tool, Zibrio offers the ability for balance and fall risk to become part of a general wellness check alongside height, weight and blood pressure. Awardee: Katharine Forth, PhD, Zibrio, Inc.
- Mabu: An AI-powered wellness coach to support rehabilitation, medication schedules, and health and wellness activities. Awardee: Cory Kidd, PhD, Catalia Health.
- CREcare: CREcare develops and implements state-of-the-art outcome measurement systems to improve the quality and efficiency of care delivery. Awardee: Alan Jette PT, MPH, PhD, Richard Moed, CREcare
- FlexVolt: A wearable muscle sensor that measures muscle activity via electromyography, offers localized vibration feedback, and wirelessly transmits data to accompanying apps providing exercises and games. Awardee: Brendan Flynn, PhD, Catabee Science and Design
- Thermoneuromodulation: TNM is a non-invasive brainstem neuromodulator that delivers heating and cooling waveforms to the vestibular system for treating symptoms of Parkinson’s disease. Awardee: Robert Black, PhD, Scion NeuroStim
- Wayband: Wayband is a wearable haptic navigation device for people who are blind and visually impaired. It guides users to an end destination using only vibration. Awardee: Keith Kirkland, MS, Wayban.
- AMBLE: AMBLE offers personalized robotic therapy to patients through adaptive software that provides a personalized assist-as-needed approach. This is the first ever clinically proven option to effectively treat foot drop. Awardee: Bradley Hennessie, MS, MBA, NextStep Robotics
- VERVE: VERVE is a virtual reality video game for rehabilitating convergence insufficiency (CI). CI is a naturally occurring binocular vision dysfunction that often manifests after traumatic brain injuries, including concussions. Awardee: Tara Alvarez, PhD, OculoMotor Technologies
- TALSA – Temple Assessment of Language & Short-term Memory in Aphasia: TALSA is a language and memory assessment for stroke or other cognitive impairments. Awardee: Nadine Martin, PhD, Temple University
- The Read Read: The Read Read™ allows a person who is blind or low-vision to independently learn Braille code using the same best-practice instruction used by teachers of the visually impaired. By touching the Braille and large print letter manipulatives, the student receives immediate audio feedback. Awardee: Alex Tavares, Ed.M., T-var EdTech, Inc.
- Limbix: Limbix is creating research-based at-home virtual reality (VR) cognitive-behavioral interventions to reduce chronic pain. Awardee: Jessica Lake, PhD, Limbix
- Evowalk: The EvoWalk device incorporates wearable sensors below the knee to detect swing motion in a person’s walk. Algorithms based on neural networks adapt to each user’s walking motion. This provides physical therapists with a robust platform to develop a range of stimulation interventions and treat a broad variety of walking styles. Awardee: Andrew Ekelem, PhD, FOOT++, Inc.
- Senaptec Quad Strobe: Senaptec Quad Strobe is eyewear designed to train the connections between an individual’s eyes, brain, and body for stroke rehabilitation. Awardee: Herb Yoo, MS, Senaptec.
- The Overcomer: The Overcomer™ Striking Device is an adaptive sport and fitness device enabling users to independently strike, swing, or throw an object they otherwise couldn’t. Awardee: Joe Kabes, Inclusivity, Inc.
- Robotic Ankle Assistive Device: A lightweight, untethered, powered ankle exoskeleton system for children with cerebral palsy. Awardee: Ray Browning, PhD, BiOMOTUM, LLC.
- SipClip: SipClip is an attachment for electric toothbrushes that interfaces with an aspirator tube. This allows people to brush their teeth and suction out fluid that may be a choking hazard. Awardee: Ashley Myers, Duke University
- Motor Assisted Uncoupled Pedaling: A device to help stroke survivors walk better that encourages paretic limb use and inter-limb coordination. Awardee: Sheila Schindler-Ivens, PT, PhD, Marquette University
- DE-AFO: DE-AFO is a comfortable smart ankle/foot orthosis designed for children with ankle control deficits to help them walk easier and longer. Awardee: Ahad Behboodi, PhD, University of Delaware
- Mio Therapy: MIO is a set of wearable smart sensors to record motion, personalize and improve outpatient rehabilitation and accelerate recovery. Awardee: Yu Suo, MbientLab Inc.
- Brain Robotics: A prosthetic hand utilizes advanced deep neural-network algorithms and multichannel sensors. Awardee: Molei Wu, PhD, BrainCo.Tech
- EDID Electronic Magnifier: A portable battery-operated magnifier for reading print with video output. Awardee: SeyedMansour Moinzadeh, Sightronics