Background
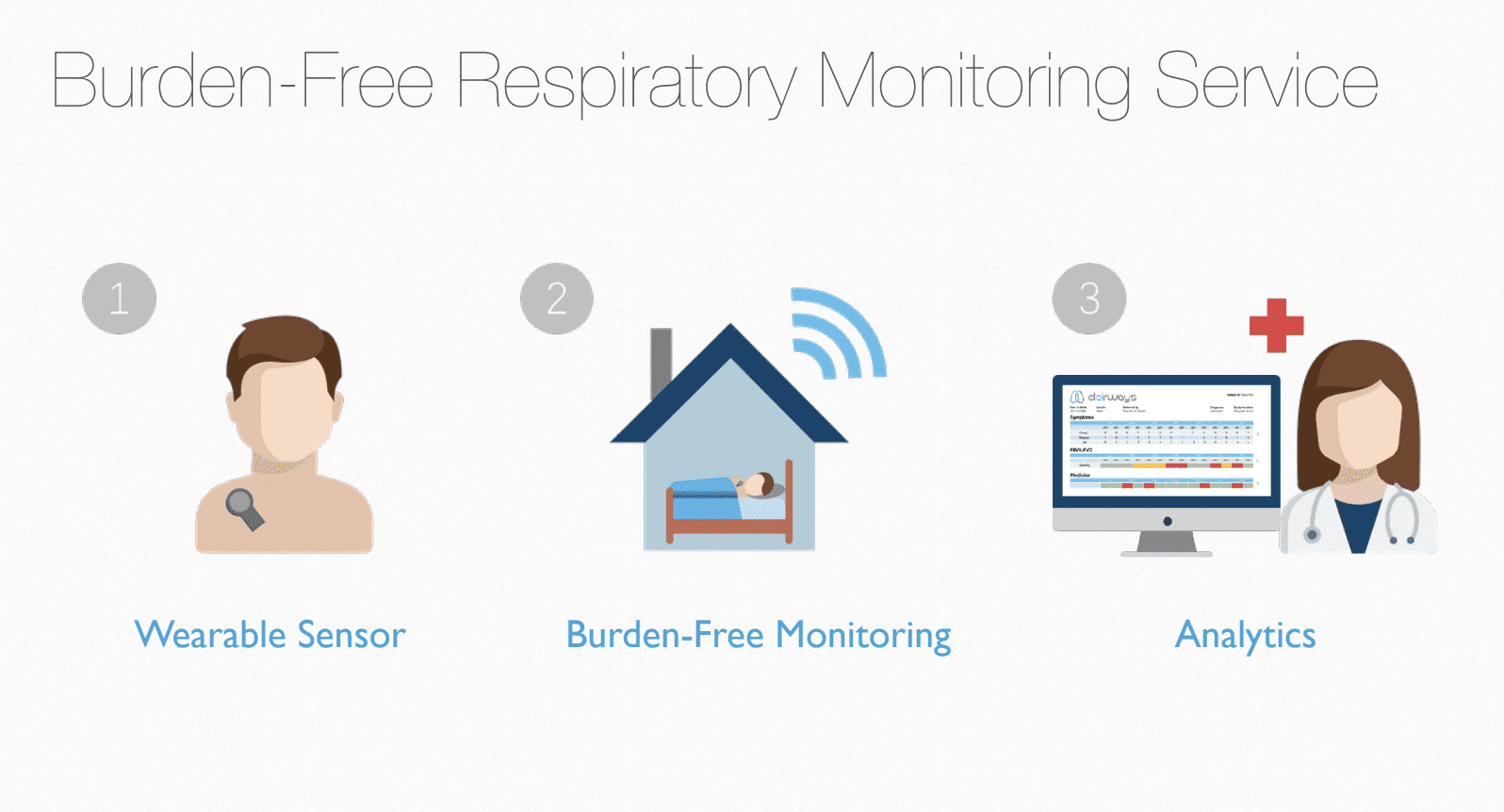
Clairways is a wearable lung monitoring device. They leverage AI to provide a comprehensive, long-term picture of respiratory health.
The AI technology detects and characterizes respiratory metrics including: coughing, wheezing, lung sounds, respiratory patterns, heart rate variability, and inhaler use. Their Ultra-Low Power Sensor allows for long-term, continuous monitoring of the lungs and airways. Clairways offers analytical reporting of trends through various metrics which are collated and reported for each patient in a clear and concise format that highlights relevant insights and patterns.
Clairways is the first place winner of the Dartmouth Pitch Competition, the J&J Quickfire Competition, and has received $150,000 from the TechOut Competition.

Challenge
“The real goal in the next year is to move from our lab functional prototype to a customer-ready device we can send home with patients,” according to Justice Amoh, Co-Founder and CTO of Clairways. Clairways is aiming to have an FDA-cleared device ready for commercialization in 2021.
As their FDA approval is being processed, Clairways is focused on identifying manufacturing and commercialization partners to bring their device to market. By conducting more respiratory trials and improving evidence, Clairways will be dramatically better positioned to garner support for commercialization. Additionally, Clairways is seeking to find the right licensee partner to assist in bringing their product to market.
Solution
Simbex assisted Clairways with the implementation of a quality management system (QMS) to improve business performance, ensure requirements and standards are maintained throughout the product lifecycle, and to meet the FDA’s Code of Federal Regulations for Quality Systems.
Additionally, Simbex worked closely with Clairways to ensure that fundraising procedures were concise and effective. These procedures included the development of a high quality pitch deck, presentation review, and enhanced lead generation.
Simbex partnered with Clairways to guide the customer interview and survey process, develop a financial forecast, evaluate potential business strategies, and guide the selection of a regulatory pathway.
The path to commercialization includes identifying customers’ problems and opportunities, designing a minimum viable product, navigating the regulatory pathway, and generating a financial plan.
Result
Through their relationship with Simbex, Clairways has been able to further develop their regulatory strategy and expand their pitch deck to target specific audiences. Conducting interviews and surveys with prospective customers and market stakeholders, Clairways was able to create a better understanding of their customer groups and business opportunities. The result of these efforts highlighted the viability of targeting the Clairways Smart Lung Monitoring system as a device intended to support respiratory clinical trials.
To accelerate commercialization, provided funds were used in electronic circuit prototyping, mechanical casing design and prototyping, and customer interviews and surveys. The prototyping work has greatly accelerated the achievement of a minimum viable product that is suitable for both customer experience exploration, as well as for IRB-approved pre-clinical studies.




